Health Library
Peripheral T-Cell Non-Hodgkin Lymphoma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
- General Information About Peripheral T-Cell Non-Hodgkin Lymphoma
- Late Effects of Treatment of Peripheral T-Cell Non-Hodgkin Lymphoma
- Cellular Classification of Peripheral T-Cell Non-Hodgkin Lymphoma
- Stage Information for Peripheral T-Cell Non-Hodgkin Lymphoma
- Treatment of Anaplastic Large Cell Lymphoma
- Treatment of Nodal Lymphomas of T Follicular Helper Cell Origin
- Treatment of Peripheral T-Cell Lymphoma, Not Otherwise Specified
- Treatment of Extranodal Natural Killer / T-Cell Lymphoma
- Treatment of Enteropathy-Associated and Monomorphic Epitheliotropic Intestinal T-Cell Lymphomas
- Treatment of Indolent T-Cell Lymphoma of the Gastrointestinal Tract
- Treatment of Hepatosplenic T-Cell Lymphoma
- Treatment of Adult T-Cell Leukemia / Lymphoma
- Treatment of T-Cell Prolymphocytic Leukemia
- Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma
- Latest Updates to This Summary (03 / 13 / 2025)
- About This PDQ Summary
General Information About Peripheral T-Cell Non-Hodgkin Lymphoma
The non-Hodgkin lymphoma (NHL) T-cell lymphomas are a heterogeneous group of T-cell lymphoproliferative malignancies, which account for less than 15% of NHLs.[1] About 85% of NHL cases are B-cell lymphomas. For more information, see Indolent B-Cell Non-Hodgkin Lymphoma Treatment and Aggressive B-Cell Non-Hodgkin Lymphoma Treatment.
T-cell lymphoma can be divided into cutaneous T-cell lymphoma (CTCL), peripheral T-cell lymphoma (PTCL), and T-cell lymphoblastic lymphoma/acute lymphocytic leukemia (T-LBL/ALL).
T-LBL/ALL arises from very early T cells, often involves the thymus, and is more common in young adults. The lymphoma form is often treated similarly to the leukemia form. For more information, see Acute Lymphoblastic Leukemia Treatment.
CTCL starts in the skin and includes mycosis fungoides, Sézary syndrome, primary cutaneous anaplastic large cell lymphoma, and others. For more information, see Mycosis Fungoides and Other Cutaneous T-Cell Lymphomas Treatment.
PTCL originates from mature T cells. It usually arises from lymphoid tissues but can spread to other organs. Subsets of PTCL include anaplastic large cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), extranodal natural killer/T-cell lymphoma (ENK/TCL), PTCL not otherwise specified (PTCL-NOS), enteropathy-associated T-cell lymphoma (EATL), hepatosplenic T-cell lymphoma (HSTCL), adult T-cell leukemia/lymphoma (ATL), T-cell prolymphocytic leukemia (T-PLL), and others.
Incidence and Mortality
T-cell lymphomas make up less than 15% of NHL cases. Most T-cell lymphoma subtypes are associated with worse outcomes than those of B-cell lymphomas.[1]
Anatomy
NHL usually originates in lymphoid tissues.
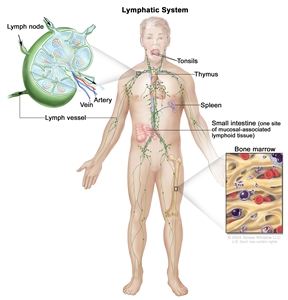
The lymph system is part of the body's immune system and is made up of tissues and organs that help protect the body from infection and disease. These include the tonsils, adenoids (not shown), thymus, spleen, bone marrow, lymph vessels, and lymph nodes. Lymph tissue is also found in many other parts of the body, including the small intestine.
Prognosis and Survival
Prognosis in PTCL varies depending on subtype, stage, and other factors. In general, PTCL is associated with a poor prognosis, with a 5-year survival rate of approximately 30% to 40%.[2,3] While outcomes are better for patients with ALK-positive ALCL, with a median 5-year overall survival (OS) closer to 70% to 80%,[2,3,4] other subsets are associated with worse survival, such as ALK-negative ALCL, AITL, PTCL-NOS, HSTCL, EATL, and ENK/TCL.[5,6]
Unlike B-cell NHLs, which include both indolent and aggressive forms, most PTCLs are considered aggressive.[7] As with most other aggressive lymphomas, PTCLs are often curable with systemic therapy, though effective treatment options are more limited, particularly in the relapsed or refractory setting.[8,9]
Even though existing treatments cure a significant fraction of patients with lymphoma, numerous clinical trials that explore treatment improvements are in progress. If possible, patients can be included in these studies.
In addition to screening for HIV among patients with aggressive lymphomas, active hepatitis B or hepatitis C can be assessed before treatment with chemotherapy.[10,11] Patients with detectable hepatitis B virus (HBV) benefit from prophylaxis with entecavir.[12,13] Patients with a resolved HBV infection (defined as hepatitis B surface antigen-negative but hepatitis B core antibody-positive) are at risk of reactivation of HBV and require monitoring of HBV DNA. Prophylactic nucleoside therapy lowered HBV reactivation from 10.8% to 2.1% in a retrospective study of 326 patients.[14] Prophylaxis for herpes zoster with acyclovir or valacyclovir and prophylaxis for pneumocystis with trimethoprim/sulfamethoxazole or dapsone are usually given to patients receiving combination chemotherapy.
References:
- American Cancer Society: Types of T-cell lymphoma. American Cancer Society, 2018. Available online. Last accessed September 13, 2024.
- Vose J, Armitage J, Weisenburger D, et al.: International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 26 (25): 4124-30, 2008.
- Ellin F, Landström J, Jerkeman M, et al.: Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 124 (10): 1570-7, 2014.
- Sibon D, Fournier M, Brière J, et al.: Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol 30 (32): 3939-46, 2012.
- Petrich AM, Helenowski IB, Bryan LJ, et al.: Factors predicting survival in peripheral T-cell lymphoma in the USA: a population-based analysis of 8802 patients in the modern era. Br J Haematol 168 (5): 708-18, 2015.
- Foss FM, Horwitz SM, Civallero M, et al.: Incidence and outcomes of rare T cell lymphomas from the T Cell Project: hepatosplenic, enteropathy associated and peripheral gamma delta T cell lymphomas. Am J Hematol 95 (2): 151-155, 2020.
- Armitage JO: The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol 92 (7): 706-715, 2017.
- Bellei M, Foss FM, Shustov AR, et al.: The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica 103 (7): 1191-1197, 2018.
- Lansigan F, Horwitz SM, Pinter-Brown LC, et al.: Outcomes for Relapsed and Refractory Peripheral T-Cell Lymphoma Patients after Front-Line Therapy from the COMPLETE Registry. Acta Haematol 143 (1): 40-50, 2020.
- Niitsu N, Hagiwara Y, Tanae K, et al.: Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol 28 (34): 5097-100, 2010.
- Dong HJ, Ni LN, Sheng GF, et al.: Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 57 (3): 209-14, 2013.
- Huang YH, Hsiao LT, Hong YC, et al.: Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 31 (22): 2765-72, 2013.
- Li H, Zhang HM, Chen LF, et al.: Prophylactic lamivudine to improve the outcome of HBsAg-positive lymphoma patients during chemotherapy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 39 (1): 80-92, 2015.
- Kusumoto S, Arcaini L, Hong X, et al.: Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 133 (2): 137-146, 2019.



