Health Library
Nasopharyngeal Carcinoma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
- General Information About Nasopharyngeal Carcinoma
- Cellular Classification of Nasopharyngeal Carcinoma
- Stage Information for Nasopharyngeal Carcinoma
- Treatment Option Overview for Nasopharyngeal Carcinoma
- Treatment of Stage I Nasopharyngeal Carcinoma
- Treatment of Stages II, III, and IV Nonmetastatic Nasopharyngeal Carcinoma
- Treatment of Metastatic and Recurrent Nasopharyngeal Carcinoma
- Latest Updates to This Summary (07 / 25 / 2024)
- About This PDQ Summary
General Information About Nasopharyngeal Carcinoma
Tumors of many histologies can occur in the nasopharynx, but only nasopharyngeal carcinomas (also called NPC) are covered in this summary. The American Joint Committee on Cancer nasopharynx staging refers exclusively to the World Health Organization's (WHO) classification of grades I, II, and III nasopharyngeal carcinoma.
Incidence and Mortality
Less than one person out of 100,000 is diagnosed with nasopharyngeal carcinoma in the world each year, with most cases found in southern China, Southeast Asia, the Arctic, and the Middle East/North Africa. The incidence is higher in males than in females.[1,2,3] WHO grade I nasopharyngeal carcinoma (keratinizing subtype) accounts for less than 20% of cases in the United States and WHO grades II and III represent the endemic form of nasopharyngeal carcinoma and are found mostly in Asia. Nonkeratinizing subtypes are associated with Epstein-Barr virus (EBV) infection and account for most cases.[4]
Anatomy
The nasopharynx has a cuboidal shape. The lateral walls are formed by the eustachian tube and the fossa of Rosenmuller. The roof, sloping downward from anterior to posterior, is bordered by the pharyngeal hypophysis, pharyngeal tonsil, and pharyngeal bursa with the base of the skull above. Anteriorly, the nasopharynx abuts the posterior choanae and nasal cavity, and the posterior boundary is formed by the muscles of the posterior pharyngeal wall. Inferiorly, the nasopharynx ends at an imaginary horizontal line formed by the upper surface of the soft palate and the posterior pharyngeal wall. Nasopharyngeal carcinoma originates from the epithelial cells that line the nasopharynx.
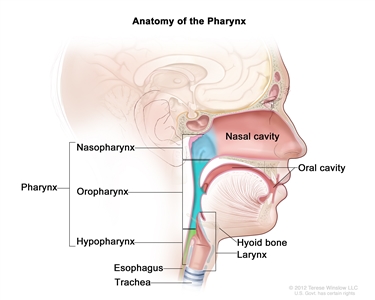
Anatomy of the pharynx.
Risk Factors
Risk factors for nasopharyngeal carcinoma include the following:[4,5,6,7,8]
Risk factors for keratinizing squamous cell carcinoma (WHO grade I):
- Heavy alcohol intake.
- History of smoking.
Risk factors for nonkeratinizing carcinoma (WHO grades II and III):
- Asian race.
- EBV exposure.
- Family history.
Clinical Features
Signs and symptoms at presentation include the following:
- Headache caused by cranial nerve dysfunction (usually II–VI or IX–XII).
- Diplopia.
- Facial numbness.
- Cervical adenopathy (present in approximately 75% of patients and often bilateral and posterior).
- Nasal obstruction.
- Epistaxis.
- Diminished hearing.
- Tinnitus.
- Otitis media.
- Sore throat.
In patients who present with cervical adenopathy alone, the finding of EBV genomic material in the tissue using polymerase chain reaction (PCR) is strong evidence of a nasopharyngeal primary tumor, and that area should be examined closely.[9]
Diagnostic Evaluation
Diagnostic tests and procedures
Diagnosis is made by biopsy of the nasopharyngeal mass. The following tests and procedures are used in the diagnosis of nasopharyngeal carcinoma:[10]
- Careful visual examination by fiberoptic nasal endoscopic examination and/or examination under anesthesia.
- Endoscopic biopsy.
- Physical examination and health history. Documentation of the size and location of the tumor and cervical lymph nodes is noted.
- Evaluation of cranial nerve function including neuro-ophthalmological evaluation and audiological evaluation.
- Computed tomography (CT) scan and/or positron emission tomography (PET)-CT scan.
- Magnetic resonance imaging (MRI) to evaluate skull base invasion.
- Circulating cancer-derived EBV DNA in plasma.[11]
- Human papillomavirus (HPV) type 16 blood test if EBV negative.
Any clinical or laboratory finding that suggests distant metastasis may prompt further evaluation of other sites. MRI is often more helpful than CT scans in assessing skull base involvement and in defining the extent of abnormalities detected.[10,12,13]
Circulating cancer-derived EBV DNA
EBV DNA in plasma samples in endemic populations may be useful in screening for early asymptomatic nasopharyngeal carcinoma. Circulating cancer-derived EBV DNA in plasma is an established tumor marker for nasopharyngeal carcinoma, with a sensitivity of 96% and a specificity of 93%.[14,15,16] The presence of short EBV DNA fragments of fewer than 181 base pairs in the plasma of nasopharyngeal carcinoma patients suggests that EBV DNA molecules are released into the circulation by apoptosis of cancer cells rather than by active viral replication.[17]
Evidence (EBV DNA in plasma for screening and diagnosis of nasopharyngeal carcinoma):
- In a study of 20,174 participants in China, EBV DNA in plasma was used to screen for early nasopharyngeal carcinoma.[14]
- Initially, 1,112 participants tested positive for EBV DNA in plasma.
- Three hundred and nine participants (1.5% of all participants, and 27.8% of those who initially tested positive) had persistently detectable EBV DNA in plasma at baseline and follow-up.
- Among the 309 participants, nasopharyngeal carcinoma was confirmed after nasal endoscopic examination, MRI, and biopsy in 34 participants (11.0%).
HPV
Differentiating HPV-related nasopharyngeal carcinoma requires identification of p16 immunohistochemical staining, in situ hybridization, and/or PCR similar to the method for differentiating HPV-related oropharyngeal cancer. Less than 10% of nonkeratinizing nasopharyngeal carcinomas are associated with HPV infection.[18,19]
Prognostic Factors
Major prognostic factors that adversely influence treatment outcome include the following:[20]
- WHO grade I.
- A higher tumor (T) stage.
- The presence of involved cervical lymph nodes (N).
- High plasma/serum EBV DNA levels before and after treatment.[21,22]
- Large tumor volume.[23][Level of evidence C1]
Follow-Up Testing and Late Effects
Follow-up testing for tumor recurrence includes the following:[24]
- Routine periodic examination of the original tumor site and neck.
- CT or PET-CT scan.
- MRI scan.
- Plasma/serum EBV DNA levels.
Patients should be monitored for the following potential late effects of treatment:[25,26]
- Xerostomia.
- Dental and oral complications.
- Hearing loss.
- Vision loss.
- Dysphagia.
- Trismus.
- Thyroid and pituitary function.
- Cranial neuropathies.
- Cognitive impairment.
Although most recurrences occur within 5 years of diagnosis, relapse can be seen at longer intervals. The incidence of second primary malignancies after treatment is lower for nasopharyngeal carcinoma than for other head and neck cancer sites.[27]
Accumulating evidence has demonstrated a high incidence (>30%–40%) of hypothyroidism in patients who have received radiation therapy that delivered external-beam radiation therapy (EBRT) to the entire thyroid gland or to the pituitary gland. Thyroid-function testing of patients should be considered before therapy and as part of posttreatment follow-up.[28,29]
Careful dental and oral hygiene evaluation and therapy is particularly important before initiation of radiation treatment. Intensity-modulated radiation therapy (IMRT) results in a lower incidence of xerostomia and may provide a better quality of life than conventional three-dimensional or two-dimensional radiation therapy (2DRT).[30,31][Level of evidence A3]
Evidence (IMRT vs. 2DRT and incidence of xerostomia):
- A randomized prospective study assessed the incidence of xerostomia in patients with early-stage nasopharyngeal carcinoma treated with IMRT (n = 28) or 2DRT (n = 28).[32] Long-term toxicities were graded with the Radiation Therapy Oncology Group (RTOG) criteria.
- The incidence of grade 2 xerostomia was 20% for patients who received IMRT and 90% for patients who received 2DRT (P = .001). There was no significant difference found between the groups with the xerostomia questionnaire.
- Patients who received IMRT had lower scores for dry mouth than patients who received 2DRT.
- The overall survival rate was 82% in the IMRT group versus 54% in the 2DRT group.
- The relapse-free survival rate was 70% in the IMRT group versus 54% in the 2DRT group.
- More late complications were reported among patients in the 2DRT group.
- The phase II RTOG-0225 study tested the feasibility of IMRT in a multi-institutional setting.[33]
- The rate of grade 2 xerostomia at 1 year from start of IMRT was 13.5%.
- The rate of grades 3 and 4 xerostomia was minimal.
- Only 2 of 68 patients were reported with grade 3 xerostomia.
- None of the patients had grade 4 xerostomia.
References:
- Petersson F: Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 32 (1): 54-73, 2015.
- Ferlay J, Soerjomataram I, Dikshit R, et al.: Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136 (5): E359-86, 2015.
- Chang ET, Adami HO: The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15 (10): 1765-77, 2006.
- Chen YP, Chan ATC, Le QT, et al.: Nasopharyngeal carcinoma. Lancet 394 (10192): 64-80, 2019.
- Chien YC, Chen JY, Liu MY, et al.: Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345 (26): 1877-82, 2001.
- Chen L, Gallicchio L, Boyd-Lindsley K, et al.: Alcohol consumption and the risk of nasopharyngeal carcinoma: a systematic review. Nutr Cancer 61 (1): 1-15, 2009.
- Okekpa SI, S M N Mydin RB, Mangantig E, et al.: Nasopharyngeal Carcinoma (NPC) Risk Factors: A Systematic Review and Meta-Analysis of the Association with Lifestyle, Diets, Socioeconomic and Sociodemographic in Asian Region. Asian Pac J Cancer Prev 20 (11): 3505-3514, 2019.
- Xie SH, Yu IT, Tse LA, et al.: Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: a case-referent study in Hong Kong Chinese. Cancer Causes Control 26 (6): 913-21, 2015.
- Feinmesser R, Miyazaki I, Cheung R, et al.: Diagnosis of nasopharyngeal carcinoma by DNA amplification of tissue obtained by fine-needle aspiration. N Engl J Med 326 (1): 17-21, 1992.
- Cummings CW, Fredrickson JM, Harker LA, et al.: Otolaryngology - Head and Neck Surgery. Mosby-Year Book, Inc., 1998.
- Kim KY, Le QT, Yom SS, et al.: Clinical Utility of Epstein-Barr Virus DNA Testing in the Treatment of Nasopharyngeal Carcinoma Patients. Int J Radiat Oncol Biol Phys 98 (5): 996-1001, 2017.
- Mendenhall WM, Werning JW, Pfister DG: Treatment of head and neck cancer. In: DeVita VT Jr, Lawrence TS, Rosenberg SA: Cancer: Principles and Practice of Oncology. 9th ed. Lippincott Williams & Wilkins, 2011, pp 729-80.
- Laramore GE, ed.: Radiation Therapy of Head and Neck Cancer. Springer-Verlag, 1989.
- Chan KCA, Woo JKS, King A, et al.: Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N Engl J Med 377 (6): 513-522, 2017.
- Lo YM, Chan LY, Lo KW, et al.: Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 59 (6): 1188-91, 1999.
- Leung SF, Zee B, Ma BB, et al.: Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 24 (34): 5414-8, 2006.
- Chan KC, Zhang J, Chan AT, et al.: Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res 63 (9): 2028-32, 2003.
- Huang WB, Chan JYW, Liu DL: Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: Multicenter study from an endemic area in Southern China. Cancer 124 (3): 530-536, 2018.
- Robinson M, Suh YE, Paleri V, et al.: Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: an observational study of correlation with ethnicity, histological subtype and outcome in a UK population. Infect Agent Cancer 8 (1): 30, 2013.
- Sanguineti G, Geara FB, Garden AS, et al.: Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys 37 (5): 985-96, 1997.
- Leung SF, Chan AT, Zee B, et al.: Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 98 (2): 288-91, 2003.
- Chan AT, Lo YM, Zee B, et al.: Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst 94 (21): 1614-9, 2002.
- Lee CC, Huang TT, Lee MS, et al.: Clinical application of tumor volume in advanced nasopharyngeal carcinoma to predict outcome. Radiat Oncol 5: 20, 2010.
- Cooper JS, Fu K, Marks J, et al.: Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys 31 (5): 1141-64, 1995.
- McDowell L, Corry J, Ringash J, et al.: Quality of Life, Toxicity and Unmet Needs in Nasopharyngeal Cancer Survivors. Front Oncol 10: 930, 2020.
- Fong R, Ward EC, Rumbach AF: Dysphagia after chemo-radiation for nasopharyngeal cancer: A scoping review. World J Otorhinolaryngol Head Neck Surg 6 (1): 10-24, 2020.
- Cooper JS, Scott C, Marcial V, et al.: The relationship of nasopharyngeal carcinomas and second independent malignancies based on the Radiation Therapy Oncology Group experience. Cancer 67 (6): 1673-7, 1991.
- Turner SL, Tiver KW, Boyages SC: Thyroid dysfunction following radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 31 (2): 279-83, 1995.
- Constine LS: What else don't we know about the late effects of radiation in patients treated for head and neck cancer? Int J Radiat Oncol Biol Phys 31 (2): 427-9, 1995.
- Pow EH, Kwong DL, McMillan AS, et al.: Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 66 (4): 981-91, 2006.
- Kam MK, Leung SF, Zee B, et al.: Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 25 (31): 4873-9, 2007.
- Poon DMC, Kam MKM, Johnson D, et al.: Durability of the parotid-sparing effect of intensity-modulated radiotherapy (IMRT) in early stage nasopharyngeal carcinoma: A 15-year follow-up of a randomized prospective study of IMRT versus two-dimensional radiotherapy. Head Neck 43 (6): 1711-1720, 2021.
- Lee N, Harris J, Garden AS, et al.: Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 27 (22): 3684-90, 2009.



