Health Library
Hodgkin Lymphoma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
- General Information About Hodgkin Lymphoma (HL)
- Cellular Classification of HL
- Stage Information for HL
- Treatment Option Overview for HL
- Treatment of Early Favorable Classic HL
- Treatment of Early Unfavorable Classic HL
- Treatment of Advanced Classic HL
- Treatment of Recurrent Classic HL
- Treatment of Nodular Lymphocyte–Predominant HL (NLPHL)
- Latest Updates to This Summary (02 / 12 / 2025)
- About This PDQ Summary
General Information About Hodgkin Lymphoma (HL)
Incidence and Mortality
Estimated new cases and deaths from HL in the United States in 2025:[1]
- New cases: 8,720.
- Deaths: 1,150.
Up to 90% of all newly diagnosed patients with HL can be cured with combination chemotherapy and/or radiation therapy.[2]
Anatomy
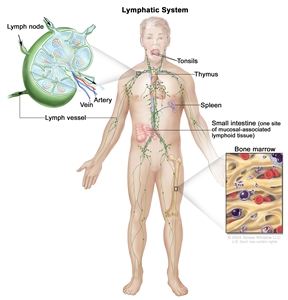
The lymph system is part of the body's immune system and is made up of tissues and organs that help protect the body from infection and disease. These include the tonsils, adenoids (not shown), thymus, spleen, bone marrow, lymph vessels, and lymph nodes. Lymph tissue is also found in many other parts of the body, including the small intestine.
HL most frequently presents in lymph node groups above the diaphragm and/or in mediastinal lymph nodes. Involvement of Waldeyer's ring or tonsillar lymph glands is rarely seen.
Risk Factors
Risk factors for HL include:
- Being in early adulthood (aged 20–39 years) (most often) or late adulthood (aged 65 years and older) (less often).
- Being male.
- Having a previous infection with the Epstein-Barr virus in the teenage years or early childhood.
- Having a first-degree relative with HL.
Clinical Features
These and other signs and symptoms may be caused by HL or by other conditions:
- Painless, swollen lymph nodes in the neck, axilla, or inguinal area.
- Fever defined as 38ºC or higher.
- Drenching and recurrent night sweats.
- Weight loss of 10% or more of baseline weight in the previous 6 months.
- Pruritus, especially after bathing or after ingesting alcohol.
- Fatigue.
Treatment of HL should relieve these symptoms within days. For more information, see Hot Flashes and Night Sweats, Pruritus, and Fatigue.
Diagnostic Evaluation
Diagnostic evaluation of patients with lymphoma may include:
- Biopsy (preferably excisional), with interpretation by a qualified pathologist.
- History, with special attention given to the presence and duration of fever, night sweats, and unexplained weight loss of 10% or more of body weight in the previous 6 months.
- Physical examination.
- Laboratory tests.
- Complete blood cell count and platelet count.
- Erythrocyte sedimentation rate.
- Chemistry panel (electrolytes, blood urea nitrogen, creatinine, calcium, aspartate transaminase, alanine aminotransferase, bilirubin, and alkaline phosphatase) plus lactate dehydrogenase, uric acid, and phosphorus.
- Radiographic examination.
- Computed tomography (CT) of the neck, chest, abdomen, and pelvis; or metabolic imaging (fluorine F 18-fludeoxyglucose positron emission tomography [PET]) with PET-CT. PET-magnetic resonance imaging scans may be equivalent to PET-CT in obtaining staging information at 25% of the radiation dose.[3]
- HIV testing.
- Hepatitis B and hepatitis C serology.
All stages of HL can be subclassified into A and B categories: B for those with defined general symptoms (described below) and A for those without B symptoms. The B designation is given to patients with any of the following symptoms:
- Unexplained weight loss (more than 10% of body weight in the 6 months before diagnosis).
- Unexplained fever with temperatures above 38°C.
- Drenching and recurrent night sweats.
The most significant B symptoms are fevers and weight loss. Night sweats alone do not confer an adverse prognosis.
Prognostic Factors
The prognosis for a given patient depends on several factors. The most important factors include:[1,4,5]
- Presence or absence of systemic B symptoms.
- Stage of disease.
- Presence of large masses.
- Quality and suitability of the treatment administered.
Other important factors are:[1,4,5]
- Age.
- Sex.
- Erythrocyte sedimentation rate.
- Hematocrit.
- Extent of abdominal involvement.
- Absolute number of nodal sites of involvement.
The best predictor of treatment failure is a PET-CT scan obtained after two cycles of chemotherapy (PET2 scan).[6,7] For limited-stage disease, there are frequent false-positive tests because the relapse risk is low (low-positive predictive value). For advanced-stage disease, up to 15% of patients have a relapse despite a negative PET2 scan (lowering the negative predictive value).[6,7] Combining biomarkers with PET-CT scanning responses or calculating metabolic tumor volume with PET-CT scanning are methods under evaluation to improve prognostic predictions.[6,8,9,10,11]
Follow-Up
Recommendations for posttreatment follow-up are not evidence based, but a variety of opinions have been published for high-risk patients who present with advanced-stage disease and for patients who achieve less-than-complete remission by PET-CT scans at the end of therapy.[12,13,14,15] For patients at high risk of relapse, conventional CT scans are used to avoid increased false-positive test results and increased radiation exposure of serial PET-CT scans.[16]
For patients with negative findings from a PET-CT scan at the end of therapy, routine scans are not advised because of the very low risk of recurrence.[17] Opportunistic scanning is applied when patients present with suspicious symptoms, physical findings, or laboratory test results. The 5-year risk of relapse from diagnosis is 5.6% for patients remaining event-free for 2 years after induction therapy.[18]
Among 6,840 patients enrolled in German Hodgkin Study Group (GHSG) trials, with a median follow-up of 10.3 years, 141 patients had a relapse after 5 years, compared with 466 patients who had a relapse within 5 years. Treatment-related adverse effects and late relapses may occur beyond 20 years of follow-up.[19]
Adverse Long-Term Effects of Therapy
Patients who complete therapy for HL are at risk of developing long-term side effects, ranging from direct damage to organ function or the immune system to second malignancies. For the first 15 years after treatment, HL is the main cause of death. By 15 to 20 years after therapy, the cumulative mortality from a second malignancy, cardiovascular disease, or pulmonary fibrosis exceeds the cumulative mortality from HL.[20,21,22,23] This risk of developing a second malignancy is even higher for individuals with a family history of cancer.[24]
Compared with the general population, long-term survivors of HL have a significantly lower life expectancy.[25] A multicenter cohort study of 4,919 patients treated between 1965 and 2000 and before age 51 years had a median follow-up of 20.2 years. Patients with HL had an absolute excess mortality (AEM) of 123 excess deaths per 10,000 person-years. This risk (standardized mortality ratio, 5.2; 95% confidence interval [CI], 4.2–6.5; AEM, 619) was maintained for 40-year survivors.[25] For example, at age 54 years, the cumulative mortality of 20.0% for HL survivors was commensurate with that of a 71-year-old person from the general population. While mortality from HL dropped precipitously from 1965 to 2000, solid tumor mortality did not change over that time.[25]
Second malignancies
Recommendations for screening for secondary malignancies or follow-up of long-term survivors are consensus based and not derived from randomized trials.[26]
Solid tumors
An increase in second solid tumors has also been observed, especially mesothelioma and cancers of the lung, breast, thyroid, bone/soft tissue, stomach, esophagus, colon and rectum, uterine cervix, and head and neck.[27,28,29,30,31,32,33,34] These tumors occur primarily after radiation therapy or with combined-modality treatment (especially when involving mechlorethamine or procarbazine), and approximately 75% occur within radiation ports. The risk of developing a second solid tumor (cumulative incidence of a second cancer) increases with time after treatment.
- At 15-years of follow-up, the risk is approximately 13%.[30]
- At 20-years of follow-up, the risk is approximately 17%.[35]
- At 25-years of follow-up, the risk is approximately 22%.[27,36]
- At 40-years of follow-up, the risk is approximately 48%.[37]
In a cohort of 18,862 5-year survivors from 13 population-based registries, the younger patients had elevated risks for breast, colon, and rectal cancers for 10 to 25 years before the ages when routine screening is recommended in the general population.[29] Even with involved-field doses of 15 Gy to 25 Gy, sarcomas, breast cancers, and thyroid cancers occurred with similar incidence in young patients, compared with those receiving higher-dose radiation.[35]
Lung cancer and breast cancer are among the most-common second solid tumors that develop after therapy for HL.
- Lung cancer. Lung cancer is seen with increased frequency, even after chemotherapy alone, and the risk of this cancer increases with cigarette smoking.[38,39,40,41] In a retrospective Surveillance, Epidemiology, and End Results (SEER) Program analysis, stage-specific survival was decreased by 30% to 60% in HL survivors, compared with patients with de novo non-small cell lung cancer.[42]
- Breast cancer. Breast cancer is seen with increased frequency after radiation therapy or combined-modality therapy.[27,28,43,44,45] The risk appears greatest for females treated with radiation before age 30 years, especially for girls close to menarche.[46] The incidence of breast cancer increases substantially after 15 years of posttherapy follow-up.[27,47,48] In a cohort of 1,964 female 5-year HL survivors treated between 1975 and 2008, doxorubicin also increased breast cancer risk independent of age at first treatment or prior chest radiation therapy.[49] Survivors who received more than 200 mg/m2 of doxorubicin had a 1.5-fold increased risk (95% CI, 1.08–2.10) versus survivors who did not receive doxorubicin.
In two case-control studies of 479 patients who developed breast cancer after therapy for HL, cumulative absolute risks for developing breast cancer were calculated as a function of radiation therapy dose and the use of chemotherapy.[50,51] With a 30-year to 40-year follow-up, cumulative absolute risks of breast cancer with exposure to radiation range from 8.5% to 39.6%, depending on age at diagnosis. These cohort studies show a continued increase in cumulative excess risk of breast cancer beyond 20 years of follow-up.[50,51]
In a nested case-control study and subsequent cohort study, patients who received both chemotherapy and radiation therapy had a statistically significant lower risk of developing breast cancer than did those treated with radiation therapy alone.[43,52] Reaching early menopause with fewer than 10 years of intact ovarian function appeared to account for the reduction in risk among patients who received combined-modality therapy.[52] Reduction of radiation volume also decreased the risk of breast cancer after HL.[52]
Late effects of autologous stem cell transplant for failure of induction chemotherapy include second malignancies, hypothyroidism, hypogonadism, herpes zoster, depression, and cardiac disease.[53]
Hematologic cancers
- Acute myelogenous leukemia (AML). Acute nonlymphocytic leukemia may occur in patients treated with combined-modality therapy or with combination chemotherapy alone, especially with increased exposure to alkylating agents.[30,54]
- At 10 years after therapy with regimens containing MOPP (mechlorethamine, vincristine, procarbazine, and prednisone), the risk of AML is approximately 3%, with the peak incidence occurring 5 to 9 years after therapy.[30,54] The risk of acute leukemia at 10 years after therapy with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) appears to be less than 1%.[55]
- A population-based study of more than 35,000 survivors during a 30-year time span identified 217 patients who developed AML. The excess absolute risk (EAR) was significantly higher for older patients (>35 years at diagnosis) than for younger survivors (EAR, 9.9 vs. 4.2 per 10,000 patient-years, P < .001).[56]
Other adverse long-term effects
Treatment of HL also affects the endocrine, cardiac, pulmonary, skeletal, and immune systems. Chronic fatigue can be a debilitating symptom for some long-term survivors.[57] A retrospective survey of 20,007 patients with early- and advanced-stage classical HL treated between 2000 and 2016 (i.e., the era in which ABVD became the preferred frontline chemotherapy regimen) showed 1,321 deaths not attributable to lymphoma (39% of total deaths). Heart disease (estimated EAR: 6.6 per 10,000 patient-years, standardized mortality ratio, 1.7 for early-stage disease and 15.1 per 10,000 patient-years, standardized mortality ratio, 2.1 for advanced-stage disease) and infection (estimated EAR: 3.1 per 10,000 patient-years, standardized mortality ratio, 2.2 for early-stage disease and 10.6 per 10,000 patient-years, standardized mortality ratio, 3.9 for advanced-stage disease) were the leading causes of death, especially in patients older than 60 years.[58]
Infertility. A toxic effect that is primarily related to chemotherapy is infertility, usually after regimens containing MOPP or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone).[59,60,61] After six to eight cycles of BEACOPP, most men had testosterone levels within reference range; however, 82% of women younger than 30 years recovered menses (mostly within 12 months), but only 45% of women older than 30 years recovered menses.[62] ABVD appears to spare long-term testicular and ovarian function.[60,63,64] Increasing age and alkylator-based regimens are the two major factors increasing the risk of premature ovarian insufficiency.[62,65,66] A prospective evaluation of gonadal function embedded in the randomized Response-Adapted Therapy in Advanced Hodgkin Lymphoma (RATHL) study for patients with newly diagnosed advanced-stage HL found good recovery of anti-Müllerian hormone concentration and reduction in follicle-stimulating hormone after ABVD or AVD (doxorubicin, vinblastine, dacarbazine), but less recovery after BEACOPP and for women older than 35 years.[65] A PET scan-adapted treatment regimen to reduce the use of BEACOPP also resulted in less infertility and gonadal dysfunction.[67] While cryopreservation of oocytes or sperm remains the first choice for preservation of fertility, luteinizing hormone-releasing hormone agonists can be tried in this setting, although efficacy for patients with HL has not been confirmed as has been confirmed for patients with breast cancer.[68] A national Danish registry of 793 HL survivors showed that patients who did not have a relapse had similar parenthood rates to the general population, but assistive reproduction methods were required more often for HL survivors (male, 21.6% vs. 6.3%; female, 13.6% vs. 5.5%; P ≤ .001 for both comparisons).[69]
Hypothyroidism. Hypothyroidism is a late complication primarily related to radiation therapy.[70,71,72] Long-term survivors who receive radiation therapy to the neck are followed up with annual thyroid-stimulating hormone testing.
Cardiac disease. A late complication primarily related to radiation therapy is cardiac disease, the risk of which may persist for over 30 years after the first treatment.[70,73,74,75,76,77,78,79,80,81] The EAR of fatal cardiovascular disease ranges from 11.9 to 48.9 per 10,000 patient-years and is mostly attributable to fatal myocardial infarction (MI).[73,74,75,77] A retrospective survey of over 6,000 patients with HL treated in trials between 1964 and 2004 found that cardiac exposure to radiation and use of doxorubicin were significant predictors of ischemic heart disease, congestive heart failure, arrhythmias, and vascular disease.[79] In a cohort of 7,033 patients with HL, MI mortality risk persisted for 25 years after first treatment with supradiaphragmatic radiation therapy (dependent on the details of treatment planning), doxorubicin, or vincristine.[77,78] A nested case-control study of 2,617 5-year survivors of HL diagnosed before age 51 years and treated between 1965 and 1995 found that the 25-year risk of moderate to severe heart failure increased for patients receiving anthracyclines. The risk ranged from 11.2% for patients exposed to 0 Gy to 15 Gy radiation up to 32.9% for patients exposed to radiation equal or greater than 21 Gy.[82] The use of subcranial blocking did not reduce the incidence of fatal MI in a retrospective review, perhaps because of the exposure of the proximal coronary arteries to radiation.[74] Compared with a general matched population, HL patients treated with mediastinal radiation were at increased risk of complications, especially during cardiac surgery.[83] Risk prediction models rely on the dose of mediastinal radiation, smoking history, male sex, and anthracycline exposure to define the patients at highest risk.[81] These risk prediction models found that mediastinal radiation therapy combined with doxorubicin exposure conferred the highest risk, followed by mediastinal radiation therapy alone.[81]
In the U.K. RAPID trial, performed between 2003 and 2010, 183 patients with early-stage HL were PET-negative but still received involved-field radiation therapy (IFRT) (20 Gy) after receiving ABVD.[80] The average predicted 30-year cardiovascular mortality was 5.02%, which included 3.52% expected in the general population, 0.94% EAR from the doxorubicin, and 0.56% from the IFRT. Since 2010, radiation therapy techniques have advanced by using smaller target volumes, lower-dose IFRT (20 Gy), deep inspiration breath holding, intensity-modulated radiation therapy, and proton beam therapy.[80] These techniques will need further evaluation to better assess cardiovascular risks from radiation therapy.
Pulmonary impairment. Impairment of pulmonary function may occur as a result of mantle-field radiation therapy; this impairment is not usually clinically evident, and recovery in pulmonary testing often occurs after 2 to 3 years.[84] Pulmonary toxic effects from bleomycin as used in ABVD are seen in patients older than 40 years.[85]
Bone necrosis. Avascular necrosis of bone has been observed in patients treated with chemotherapy and is most likely related to corticosteroid therapy.[86]
Bacterial sepsis. Bacterial sepsis may occur rarely after splenectomy performed during staging laparotomy for HL;[87] it is much more common in children than in adults.
Fatigue. Fatigue is a commonly reported symptom among patients who have completed chemotherapy and radiation therapy. In a case-control study design, most HL survivors reported significant fatigue lasting for more than 6 months after therapy, compared with age-matched controls. Quality-of-life questionnaires given to 5,306 patients on GHSG trials showed that 20% of patients complained of severe fatigue 5 years after therapy, and those patients had significantly increased problems with employment and financial stability.[88,89,90] For more information, see Fatigue.
Neurocognitive impairment. After a median of 23 years from diagnosis, 1,760 HL survivors treated in childhood were compared with 3,180 siblings. Significantly higher rates of memory loss (8.1% vs. 5.7%; P < .05), anxiety (7.0% vs. 5.4%; P < .05), unemployment (9.6% vs. 4.4%; P < .05), depression (9.1% vs. 7.0%; P < .05), and impaired physical quality of life (11.2% vs. 3.0%; P < .05) were reported.[91] Lower risks were associated with survivors who adhered to exercise guidelines and did not smoke, but the design of this study did not allow a cause-and-effect conclusion.
References:
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Brice P, de Kerviler E, Friedberg JW: Classical Hodgkin lymphoma. Lancet 398 (10310): 1518-1527, 2021.
- Picardi M, Cavaliere C, Della Pepa R, et al.: PET/MRI for staging patients with Hodgkin lymphoma: equivalent results with PET/CT in a prospective trial. Ann Hematol 100 (6): 1525-1535, 2021.
- Cosset JM, Henry-Amar M, Meerwaldt JH, et al.: The EORTC trials for limited stage Hodgkin's disease. The EORTC Lymphoma Cooperative Group. Eur J Cancer 28A (11): 1847-50, 1992.
- Evens AM, Helenowski I, Ramsdale E, et al.: A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood 119 (3): 692-5, 2012.
- Agostinelli C, Gallamini A, Stracqualursi L, et al.: The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin's lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol 3 (10): e467-e479, 2016.
- Gallamini A, Rossi A, Patti C, et al.: Interim PET-adapted chemotherapy in advanced Hodgkin lymphoma: results of the second interim analysis of the Italian GITIL/FIL DH0607 trial. [Abstract] Hematol Oncol 33 (Suppl 1): A-118, 100-180, 2015.
- Spina V, Bruscaggin A, Cuccaro A, et al.: Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood 131 (22): 2413-2425, 2018.
- Cottereau AS, Versari A, Loft A, et al.: Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 131 (13): 1456-1463, 2018.
- Akhtari M, Milgrom SA, Pinnix CC, et al.: Reclassifying patients with early-stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood 131 (1): 84-94, 2018.
- Moskowitz AJ, Schöder H, Gavane S, et al.: Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood 130 (20): 2196-2203, 2017.
- Hoppe RT, Advani RH, Ai WZ, et al.: Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw 10 (5): 589-97, 2012.
- Ng A, Constine LS, Advani R, et al.: ACR Appropriateness Criteria: follow-up of Hodgkin's lymphoma. Curr Probl Cancer 34 (3): 211-27, 2010 May-Jun.
- Armitage JO: Who benefits from surveillance imaging? J Clin Oncol 30 (21): 2579-80, 2012.
- Picardi M, Pugliese N, Cirillo M, et al.: Advanced-stage Hodgkin lymphoma: US/chest radiography for detection of relapse in patients in first complete remission--a randomized trial of routine surveillance imaging procedures. Radiology 272 (1): 262-74, 2014.
- El-Galaly TC, Mylam KJ, Brown P, et al.: Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica 97 (6): 931-6, 2012.
- Hartridge-Lambert SK, Schöder H, Lim RC, et al.: ABVD alone and a PET scan complete remission negates the need for radiologic surveillance in early-stage, nonbulky Hodgkin lymphoma. Cancer 119 (6): 1203-9, 2013.
- Hapgood G, Zheng Y, Sehn LH, et al.: Evaluation of the Risk of Relapse in Classical Hodgkin Lymphoma at Event-Free Survival Time Points and Survival Comparison With the General Population in British Columbia. J Clin Oncol 34 (21): 2493-500, 2016.
- Bröckelmann PJ, Goergen H, Kohnhorst C, et al.: Late Relapse of Classical Hodgkin Lymphoma: An Analysis of the German Hodgkin Study Group HD7 to HD12 Trials. J Clin Oncol 35 (13): 1444-1450, 2017.
- Mauch PM, Kalish LA, Marcus KC, et al.: Long-Term Survival in Hodgkin's Disease Cancer J Sci Am 1 (1): 33-42, 1995.
- Aisenberg AC: Problems in Hodgkin's disease management. Blood 93 (3): 761-79, 1999.
- Longo DL, Armitage JO: Controversies in the treatment of early-stage Hodgkin's lymphoma. N Engl J Med 372 (17): 1667-9, 2015.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al.: Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 21 (18): 3431-9, 2003.
- Sud A, Thomsen H, Sundquist K, et al.: Risk of Second Cancer in Hodgkin Lymphoma Survivors and Influence of Family History. J Clin Oncol 35 (14): 1584-1590, 2017.
- de Vries S, Schaapveld M, Janus CPM, et al.: Long-Term Cause-Specific Mortality in Hodgkin Lymphoma Patients. J Natl Cancer Inst 113 (6): 760-769, 2021.
- Ng AK: Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood 124 (23): 3373-9, 2014.
- Dores GM, Metayer C, Curtis RE, et al.: Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol 20 (16): 3484-94, 2002.
- Franklin J, Pluetschow A, Paus M, et al.: Second malignancy risk associated with treatment of Hodgkin's lymphoma: meta-analysis of the randomised trials. Ann Oncol 17 (12): 1749-60, 2006.
- Hodgson DC, Gilbert ES, Dores GM, et al.: Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 25 (12): 1489-97, 2007.
- Swerdlow AJ, Higgins CD, Smith P, et al.: Second cancer risk after chemotherapy for Hodgkin's lymphoma: a collaborative British cohort study. J Clin Oncol 29 (31): 4096-104, 2011.
- Chowdhry AK, McHugh C, Fung C, et al.: Second primary head and neck cancer after Hodgkin lymphoma: a population-based study of 44,879 survivors of Hodgkin lymphoma. Cancer 121 (9): 1436-45, 2015.
- Dores GM, Curtis RE, van Leeuwen FE, et al.: Pancreatic cancer risk after treatment of Hodgkin lymphoma. Ann Oncol 25 (10): 2073-9, 2014.
- Rigter LS, Spaander MCW, Aleman BMP, et al.: High prevalence of advanced colorectal neoplasia and serrated polyposis syndrome in Hodgkin lymphoma survivors. Cancer 125 (6): 990-999, 2019.
- Geurts YM, Shakir R, Ntentas G, et al.: Association of Radiation and Procarbazine Dose With Risk of Colorectal Cancer Among Survivors of Hodgkin Lymphoma. JAMA Oncol 9 (4): 481-489, 2023.
- O'Brien MM, Donaldson SS, Balise RR, et al.: Second malignant neoplasms in survivors of pediatric Hodgkin's lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol 28 (7): 1232-9, 2010.
- Bonadonna G, Viviani S, Bonfante V, et al.: Survival in Hodgkin's disease patients--report of 25 years of experience at the Milan Cancer Institute. Eur J Cancer 41 (7): 998-1006, 2005.
- Schaapveld M, Aleman BM, van Eggermond AM, et al.: Second Cancer Risk Up to 40 Years after Treatment for Hodgkin's Lymphoma. N Engl J Med 373 (26): 2499-511, 2015.
- van Leeuwen FE, Klokman WJ, Stovall M, et al.: Roles of radiotherapy and smoking in lung cancer following Hodgkin's disease. J Natl Cancer Inst 87 (20): 1530-7, 1995.
- Swerdlow AJ, Schoemaker MJ, Allerton R, et al.: Lung cancer after Hodgkin's disease: a nested case-control study of the relation to treatment. J Clin Oncol 19 (6): 1610-8, 2001.
- Travis LB, Gospodarowicz M, Curtis RE, et al.: Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst 94 (3): 182-92, 2002.
- Lorigan P, Radford J, Howell A, et al.: Lung cancer after treatment for Hodgkin's lymphoma: a systematic review. Lancet Oncol 6 (10): 773-9, 2005.
- Milano MT, Li H, Constine LS, et al.: Survival after second primary lung cancer: a population-based study of 187 Hodgkin lymphoma patients. Cancer 117 (24): 5538-47, 2011.
- van Leeuwen FE, Klokman WJ, Stovall M, et al.: Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst 95 (13): 971-80, 2003.
- Wahner-Roedler DL, Nelson DF, Croghan IT, et al.: Risk of breast cancer and breast cancer characteristics in women treated with supradiaphragmatic radiation for Hodgkin lymphoma: Mayo Clinic experience. Mayo Clin Proc 78 (6): 708-15, 2003.
- Travis LB, Hill DA, Dores GM, et al.: Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA 290 (4): 465-75, 2003.
- Moskowitz CS, Ronckers CM, Chou JF, et al.: Development and Validation of a Breast Cancer Risk Prediction Model for Childhood Cancer Survivors Treated With Chest Radiation: A Report From the Childhood Cancer Survivor Study and the Dutch Hodgkin Late Effects and LATER Cohorts. J Clin Oncol 39 (27): 3012-3021, 2021.
- Alm El-Din MA, Hughes KS, Finkelstein DM, et al.: Breast cancer after treatment of Hodgkin's lymphoma: risk factors that really matter. Int J Radiat Oncol Biol Phys 73 (1): 69-74, 2009.
- Cooke R, Jones ME, Cunningham D, et al.: Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br J Cancer 108 (11): 2399-406, 2013.
- Neppelenbroek SIM, Geurts YM, Aleman BMP, et al.: Doxorubicin Exposure and Breast Cancer Risk in Survivors of Adolescent and Adult Hodgkin Lymphoma. J Clin Oncol 42 (16): 1903-1913, 2024.
- Travis LB, Hill D, Dores GM, et al.: Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst 97 (19): 1428-37, 2005.
- Swerdlow AJ, Cooke R, Bates A, et al.: Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin's lymphoma in England and Wales: a National Cohort Study. J Clin Oncol 30 (22): 2745-52, 2012.
- De Bruin ML, Sparidans J, van't Veer MB, et al.: Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27 (26): 4239-46, 2009.
- Lavoie JC, Connors JM, Phillips GL, et al.: High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood 106 (4): 1473-8, 2005.
- Koontz MZ, Horning SJ, Balise R, et al.: Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol 31 (5): 592-8, 2013.
- Valagussa P, Santoro A, Fossati-Bellani F, et al.: Second acute leukemia and other malignancies following treatment for Hodgkin's disease. J Clin Oncol 4 (6): 830-7, 1986.
- Schonfeld SJ, Gilbert ES, Dores GM, et al.: Acute myeloid leukemia following Hodgkin lymphoma: a population-based study of 35,511 patients. J Natl Cancer Inst 98 (3): 215-8, 2006.
- Kreissl S, Müller H, Goergen H, et al.: Health-Related Quality of Life in Patients With Hodgkin Lymphoma: A Longitudinal Analysis of the German Hodgkin Study Group. J Clin Oncol 38 (25): 2839-2848, 2020.
- Dores GM, Curtis RE, Dalal NH, et al.: Cause-Specific Mortality Following Initial Chemotherapy in a Population-Based Cohort of Patients With Classical Hodgkin Lymphoma, 2000-2016. J Clin Oncol 38 (35): 4149-4162, 2020.
- Behringer K, Breuer K, Reineke T, et al.: Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol 23 (30): 7555-64, 2005.
- van der Kaaij MA, Heutte N, Le Stang N, et al.: Gonadal function in males after chemotherapy for early-stage Hodgkin's lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 25 (19): 2825-32, 2007.
- Scholz M, Engert A, Franklin J, et al.: Impact of first- and second-line treatment for Hodgkin's lymphoma on the incidence of AML/MDS and NHL--experience of the German Hodgkin's Lymphoma Study Group analyzed by a parametric model of carcinogenesis. Ann Oncol 22 (3): 681-8, 2011.
- Behringer K, Mueller H, Goergen H, et al.: Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol 31 (2): 231-9, 2013.
- Viviani S, Santoro A, Ragni G, et al.: Pre- and post-treatment testicular dysfunction in Hodgkin's disease (HD). [Abstract] Proceedings of the American Society of Clinical Oncology 7: A-877, 227, 1988.
- van der Kaaij MA, Heutte N, Meijnders P, et al.: Premature ovarian failure and fertility in long-term survivors of Hodgkin's lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol 30 (3): 291-9, 2012.
- Anderson RA, Remedios R, Kirkwood AA, et al.: Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin's lymphoma (RATHL): a secondary analysis of a randomised phase 3 trial. Lancet Oncol 19 (10): 1328-1337, 2018.
- Weibull CE, Johansson ALV, Eloranta S, et al.: Contemporarily Treated Patients With Hodgkin Lymphoma Have Childbearing Potential in Line With Matched Comparators. J Clin Oncol 36 (26): 2718-2725, 2018.
- Demeestere I, Racape J, Dechene J, et al.: Gonadal Function Recovery in Patients With Advanced Hodgkin Lymphoma Treated With a PET-Adapted Regimen: Prospective Analysis of a Randomized Phase III Trial (AHL2011). J Clin Oncol 39 (29): 3251-3260, 2021.
- Lambertini M, Demeestere I: Another step towards improving oncofertility counselling of young women with Hodgkin's lymphoma. Lancet Oncol 19 (10): 1264-1266, 2018.
- Øvlisen AK, Jakobsen LH, Eloranta S, et al.: Parenthood Rates and Use of Assisted Reproductive Techniques in Younger Hodgkin Lymphoma Survivors: A Danish Population-Based Study. J Clin Oncol 39 (31): 3463-3472, 2021.
- Tarbell NJ, Thompson L, Mauch P: Thoracic irradiation in Hodgkin's disease: disease control and long-term complications. Int J Radiat Oncol Biol Phys 18 (2): 275-81, 1990.
- Hancock SL, Cox RS, McDougall IR: Thyroid diseases after treatment of Hodgkin's disease. N Engl J Med 325 (9): 599-605, 1991.
- Cella L, Conson M, Caterino M, et al.: Thyroid V30 predicts radiation-induced hypothyroidism in patients treated with sequential chemo-radiotherapy for Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys 82 (5): 1802-8, 2012.
- Reinders JG, Heijmen BJ, Olofsen-van Acht MJ, et al.: Ischemic heart disease after mantlefield irradiation for Hodgkin's disease in long-term follow-up. Radiother Oncol 51 (1): 35-42, 1999.
- Hancock SL, Tucker MA, Hoppe RT: Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 270 (16): 1949-55, 1993.
- Heidenreich PA, Schnittger I, Strauss HW, et al.: Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol 25 (1): 43-9, 2007.
- Dabaja B, Cox JD, Buchholz TA: Radiation therapy can still be used safely in combined modality approaches in patients with Hodgkin's lymphoma. J Clin Oncol 25 (1): 3-5, 2007.
- Swerdlow AJ, Higgins CD, Smith P, et al.: Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 99 (3): 206-14, 2007.
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al.: Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol 34 (3): 235-43, 2016.
- Maraldo MV, Giusti F, Vogelius IR, et al.: Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2 (11): e492-502, 2015.
- Cutter DJ, Ramroth J, Diez P, et al.: Predicted Risks of Cardiovascular Disease Following Chemotherapy and Radiotherapy in the UK NCRI RAPID Trial of Positron Emission Tomography-Directed Therapy for Early-Stage Hodgkin Lymphoma. J Clin Oncol 39 (32): 3591-3601, 2021.
- de Vries S, Haaksma ML, Jóźwiak K, et al.: Development and Validation of Risk Prediction Models for Coronary Heart Disease and Heart Failure After Treatment for Hodgkin Lymphoma. J Clin Oncol 41 (1): 86-95, 2023.
- van Nimwegen FA, Ntentas G, Darby SC, et al.: Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 129 (16): 2257-2265, 2017.
- Galper SL, Yu JB, Mauch PM, et al.: Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 117 (2): 412-8, 2011.
- Horning SJ, Adhikari A, Rizk N, et al.: Effect of treatment for Hodgkin's disease on pulmonary function: results of a prospective study. J Clin Oncol 12 (2): 297-305, 1994.
- Martin WG, Ristow KM, Habermann TM, et al.: Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol 23 (30): 7614-20, 2005.
- Prosnitz LR, Lawson JP, Friedlaender GE, et al.: Avascular necrosis of bone in Hodgkin's disease patients treated with combined modality therapy. Cancer 47 (12): 2793-7, 1981.
- Schimpff SC, O'Connell MJ, Greene WH, et al.: Infections in 92 splenectomized patients with Hodgkin's disease. A clinical review. Am J Med 59 (5): 695-701, 1975.
- Behringer K, Goergen H, Müller H, et al.: Cancer-Related Fatigue in Patients With and Survivors of Hodgkin Lymphoma: The Impact on Treatment Outcome and Social Reintegration. J Clin Oncol 34 (36): 4329-4337, 2016.
- Loge JH, Abrahamsen AF, Ekeberg O, et al.: Hodgkin's disease survivors more fatigued than the general population. J Clin Oncol 17 (1): 253-61, 1999.
- Kreissl S, Mueller H, Goergen H, et al.: Cancer-related fatigue in patients with and survivors of Hodgkin's lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol 17 (10): 1453-1462, 2016.
- Williams AM, Mirzaei Salehabadi S, Xing M, et al.: Modifiable risk factors for neurocognitive and psychosocial problems after Hodgkin lymphoma. Blood 139 (20): 3073-3086, 2022.



