Health Library
Chronic Myelogenous Leukemia Treatment (PDQ®): Treatment - Health Professional Information [NCI]
- General Information About Chronic Myeloid Leukemia (CML)
- Histopathology and Phases of CML
- Treatment Option Overview for CML
- Treatment of Chronic-Phase CML
- Treatment of Accelerated-Phase CML
- Treatment of Blastic-Phase CML
- Treatment of Relapsed CML
- Key References for CML
- Latest Updates to This Summary (03 / 13 / 2025)
- About This PDQ Summary
General Information About Chronic Myeloid Leukemia (CML)
Incidence and Mortality
Estimated new cases and deaths from CML in the United States in 2025:[1]
- New cases: 9,560.
- Deaths: 1,290.
CML is one of a group of diseases called the myeloproliferative disorders. It is also called chronic myelogenous leukemia. Other related entities include:
- Polycythemia vera.
- Myelofibrosis.
- Essential thrombocythemia.
For more information, see Myeloproliferative Neoplasms Treatment.
Molecular Genetics
CML is identified by too many myeloblasts in the blood and bone marrow, and the disease worsens as the number of myeloblasts increase.
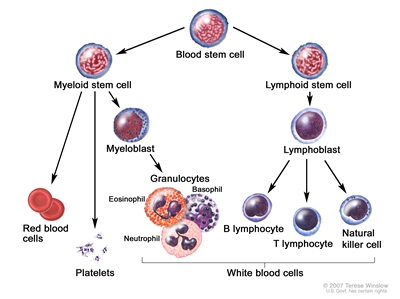
Figure 1. Hematopoietic tree, expanded lymphoid line.
CML is a clonal disorder that is easily diagnosed because the leukemic cells of more than 95% of patients have a distinctive cytogenetic abnormality, the Philadelphia chromosome (Ph).[2]
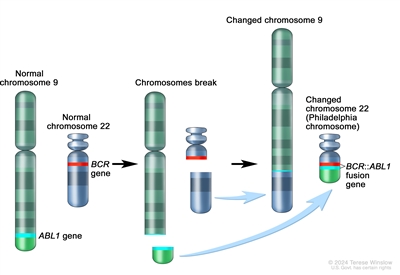
Figure 2. The Philadelphia chromosome is a translocation between the ABL1 oncogene (on the long arm of chromosome 9) and the BCR gene (on the long arm of chromosome 22), resulting in the BCR::ABL1 fusion gene. BCR::ABL1 encodes an oncogenic protein with tyrosine kinase activity.
The Ph chromosome results from a reciprocal translocation between the long arms of chromosomes 9 and 22, and it is demonstrable in all hematopoietic precursors.[3] This translocation results in the transfer of the ABL1 oncogene on chromosome 9 to an area of chromosome 22 termed the breakpoint cluster region (within the BCR gene).[3] This, in turn, results in a BCR::ABL1 fusion gene and in the production of an abnormal tyrosine kinase protein that causes the disordered myelopoiesis found in CML. Using peripheral blood, molecular techniques can detect the presence of the 9;22 translocation.
Clinical Presentation
Although CML may present without symptoms, splenomegaly is the most common finding during physical examination at the time of diagnosis.[4] The spleen may be enormous, filling most of the abdomen, causing pain or a feeling of fullness and presenting a significant clinical problem, or the spleen may be only minimally enlarged. In about 10% of patients, the spleen is neither palpable nor enlarged on computed tomography (CT) scan.
Patients may also present with the following symptoms:
- Fatigue.
- Unexplained weight loss.
- Drenching night sweats.
- Fever.
Transition between the chronic, accelerated, and blastic phases may occur gradually over 1 year or more, or it may occur abruptly (blast crisis). Patients with accelerated-phase CML show signs of progression without meeting the criteria for blast crisis (acute leukemia). The following signs and symptoms indicate a change to accelerated-phase CML:
- Progressive splenomegaly.
- Increased leukocytosis and/or thrombocytosis.
- Progressive anemia.
The following signs and symptoms indicate a change to a blast crisis, in addition to the accelerated-phase CML symptoms:
- Thrombocytopenia.
- Increasing and painful splenomegaly or hepatomegaly.
- Fever.
- Bone pain.
- Development of destructive bone lesions.
In the accelerated phase, differentiated cells persist, although they often show increasing morphological abnormalities. The patient experiences increased anemia, thrombocytopenia, and marrow fibrosis.[4]
Risk Factors
Risk factors for CML include:
- Older age.
- Exposure to high-dose ionizing radiation.
Diagnostic Evaluation
In addition to a health history and physical examination, the initial workup may include:
- Complete blood count with differential.
- Blood chemistry studies.
- Bone marrow aspiration and biopsy. In routine presentations of CML, the utility of bone marrow aspiration and biopsy for all newly diagnosed patients is questionable outside the context of a clinical trial. Bone marrow testing is appropriate for patients with clinical signs of accelerated phase or blast crisis (fever, enlarged spleen, or >20% blasts in the peripheral blood).[5]
- Cytogenetic analysis.
- Fluorescence in situ hybridization (FISH). FISH of the BCR::ABL1 translocation can be performed using the bone marrow aspirate or peripheral blood of patients with CML.[4]
- Reverse transcription–polymerase chain reaction (RT-PCR). A small subset of patients has the BCR::ABL1 rearrangement detectable only by RT-PCR, which is the most sensitive technique currently available. Patients with RT-PCR evidence of the BCR::ABL1 fusion gene appear clinically and prognostically identical to patients with a classic Ph chromosome. However, patients who are BCR::ABL1-negative by RT-PCR have a clinical course more consistent with chronic myelomonocytic leukemia, which is a distinct clinical entity related to myelodysplastic syndrome.[6,7,8]
- CT scan.
Prognosis and Survival
The median age of patients with Ph chromosome–positive CML is 67 years.[9] With the advent of the oral tyrosine kinase inhibitors (TKIs) , the median survival is projected to approach normal life expectancy for most patients.[10]
Ph chromosome–negative CML is a poorly defined entity that is less clearly distinguished from other myeloproliferative syndromes. Patients with Ph chromosome–negative CML generally have a poorer response to treatment and shorter survival than Ph chromosome–positive patients.[11] Ph chromosome–negative patients who have BCR::ABL1 gene rearrangements detectable by Southern blot analysis, however, have prognoses equivalent to Ph chromosome–positive patients.[6,12]
References:
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Jabbour E, Kantarjian H: Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol 95 (6): 691-709, 2020.
- Deininger MW, Goldman JM, Melo JV: The molecular biology of chronic myeloid leukemia. Blood 96 (10): 3343-56, 2000.
- Jabbour E, Kantarjian H: Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol 87 (11): 1037-45, 2012.
- Hidalgo-Lόpez JE, Kanagal-Shamanna R, Quesada AE, et al.: Bone marrow core biopsy in 508 consecutive patients with chronic myeloid leukemia: Assessment of potential value. Cancer 124 (19): 3849-3855, 2018.
- Martiat P, Michaux JL, Rodhain J: Philadelphia-negative (Ph-) chronic myeloid leukemia (CML): comparison with Ph+ CML and chronic myelomonocytic leukemia. The Groupe Français de Cytogénétique Hématologique. Blood 78 (1): 205-11, 1991.
- Oscier DG: Atypical chronic myeloid leukaemia, a distinct clinical entity related to the myelodysplastic syndrome? Br J Haematol 92 (3): 582-6, 1996.
- Kurzrock R, Bueso-Ramos CE, Kantarjian H, et al.: BCR rearrangement-negative chronic myelogenous leukemia revisited. J Clin Oncol 19 (11): 2915-26, 2001.
- Lee SJ, Anasetti C, Horowitz MM, et al.: Initial therapy for chronic myelogenous leukemia: playing the odds. J Clin Oncol 16 (9): 2897-903, 1998.
- Bower H, Björkholm M, Dickman PW, et al.: Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol 34 (24): 2851-7, 2016.
- Onida F, Ball G, Kantarjian HM, et al.: Characteristics and outcome of patients with Philadelphia chromosome negative, bcr/abl negative chronic myelogenous leukemia. Cancer 95 (8): 1673-84, 2002.
- Cortes JE, Talpaz M, Beran M, et al.: Philadelphia chromosome-negative chronic myelogenous leukemia with rearrangement of the breakpoint cluster region. Long-term follow-up results. Cancer 75 (2): 464-70, 1995.



