Health Library
Childhood Thyroid Cancer Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Childhood Thyroid Cancer
Incidence
In the United States, the annual incidence of thyroid cancers is 10.7 cases per 1 million in people aged 0 to 19 years. The incidence is higher in females than in males (17.6 vs. 4.1 cases per 1 million people, respectively) and lower in Black people than in White people (3.9 vs. 11.8 cases per 1 million people, respectively). It accounts for approximately 6% of all cancers in this age group.[1] Thyroid cancer incidence is higher in children aged 15 to 19 years (34.4 cases per 1 million people), and it accounts for approximately 14% of all cancers arising in this older age group.[1] The trend toward larger tumors suggests that diagnostic scrutiny is not the only explanation for the observed results.[2]
Two time-trend studies using the Surveillance, Epidemiology, and End Results (SEER) Program database have shown a 2% and 3.8% annual increase in the incidence of differentiated thyroid carcinoma in the United States among children, adolescents, and young adults in the 1973 to 2011 and 1984 to 2010 periods, respectively.[2,3] Newer data from the National Childhood Cancer Registry show an average annual increase in incidence rates of 1.2% between 2012 and 2021, without changes in survival.[1] A similar trend has been documented in other countries.[4,5]
The papillary subtype is the most common subtype of childhood thyroid cancer, accounting for approximately 60% of cases, followed by the papillary follicular variant subtype (20%–25%), the follicular subtype (10%), and the medullary subtype (<10%). The anaplastic subtype occurs in less than 1% of pediatric thyroid carcinomas. The incidence of the papillary subtype and its follicular variant peaks between the ages of 15 and 19 years. The incidence of medullary thyroid cancer is the highest in children aged 0 to 4 years and declines at older ages (see Figure 1).[6]
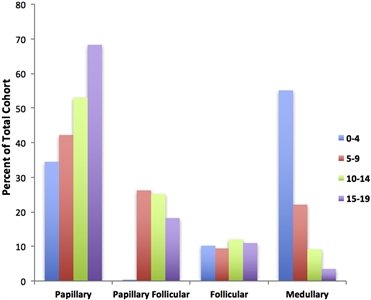
Figure 1. Incidence of pediatric thyroid carcinoma based on most frequent subtype per 100,000 as a percent of total cohort. Reprinted from International Journal of Pediatric Otorhinolaryngology, Volume 89, Sarah Dermody, Andrew Walls, Earl H. Harley Jr., Pediatric thyroid cancer: An update from the SEER database 2007–2012, Pages 121–126, Copyright (2016), with permission from Elsevier.
Diagnostic Evaluation
The prevalence of benign thyroid nodules in childhood has been estimated at about 0.5% to 2%.[7] However, thyroid nodules in children have a higher risk of malignancy (22%–26%) than thyroid nodules in adults (5%–15%).[8] Initial evaluation of a child or adolescent with a thyroid nodule includes the following:
- Ultrasonography of the thyroid and neck. Common ultrasonographic features of malignancy include hypoechogenicity, invasive margins, increased intranodular blood flow, microcalcifications, and abnormal cervical lymph nodes. Based on ultrasonographic characteristics, scoring systems have been developed to facilitate selection of nodules that require fine-needle aspiration (FNA) in adults. The most popular of these scoring systems is the Thyroid Imaging Reporting and Data System. However, the higher incidence of differentiated thyroid carcinoma in pediatric thyroid nodules and the lack of validation in the pediatric population limits the extrapolation of these criteria to children.[7,8]
- Serum thyroid-stimulating hormone (TSH) level. Thyroid function is usually normal. Hyperfunctioning nodules have a very low risk of malignancy (2%–6%).[8]
- Serum thyroglobulin level, which is usually elevated in differentiated thyroid carcinoma.
- FNA. The sensitivity, specificity, and accuracy of FNA in children are similar to those in adults, but there is a greater risk of false-negative findings in nodules larger than 4 cm.[8]
FNA results are categorized according to the six tiers of The Bethesda System for Reporting Thyroid Cytopathology (see Table 1).[8]
Table 1. Bethesda System for Reporting Thyroid Cytopathologya Bethesda Category Cytopathological Category Malignancy Rate Suggested Treatment FNA = fine-needle aspiration; US = ultrasonography. a Reprinted fromJournal of Pediatric Surgery, Volume 55, Issue 11, Emily R. Christison-Lagay, Reto M. Baertschiger, Catherine Dinauer, Gary L. Francis, Marcus M. Malek, Timothy B Lautz, Jennifer H. Aldrink, Christa Grant, Daniel S. Rhee, Peter Ehrlich, Roshni Dasgupta, Shahab Abdessalam, Pediatric differentiated thyroid carcinoma: An update from the APSA Cancer Committee, Pages 2273–2283, Copyright (2020), with permission from Elsevier.[8] I Nondiagnostic/inadequate 1%–5% Repeat FNA (other options: continued US surveillance, lobectomy) II Benign 0%–10% Serial US if small, lobectomy if >4 cm III Atypia/follicular lesion of undetermined significance 0%–44% Molecular genetics, lobectomy if no concerning mutation, thyroidectomy ifBRAFor fusion mutation IV Follicular neoplasm 60%–71% Molecular genetics, lobectomy if no concerning mutation, thyroidectomy ifBRAFor fusion mutation V Suspicious for malignancy 70%–86% Total thyroidectomy +/− central neck dissection VI Malignant 97%–100% Total thyroidectomy +/− central neck dissection While molecular testing of thyroid nodules could be helpful in the diagnosis of papillary thyroid carcinoma, there is no evidence to support its use.[7]
- Lymph node evaluation. Examination of the cervical lymph nodes is critically important in stratifying risk and determining operative strategies. Architecturally concerning features found on ultrasound in adults include round shape, irregular margins, calcifications, cystic change, peripheral vascularity, loss of fatty hilum, and heterogeneous echotexture. FNA should be performed on any suspicious lymph nodes in the lateral neck as confirmation of metastatic involvement before lateral neck dissection.[8]
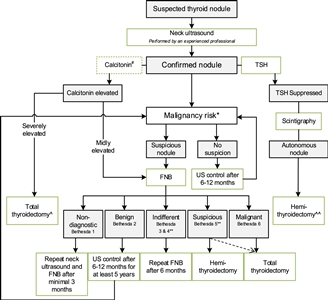
Figure 2. Flowchart showing the initial evaluation, treatment, and follow-up of pediatric thyroid nodules. #The expert panel suggests considering the measurement of serum calcitonin in children suspect of medullary thyroid carcinoma (MTC) based on individual conditions and the preference of the physician (Recommendation 5A). The expert panel suggests that, in selected cases (conditions that suggest MEN2, a positive family history of MEN2, or in case of bulky thyroid disease), the measurement of calcitonin may be of additional value for early diagnosis of MTC (Recommendation 5B). *Malignancy risk (suspicious vs. no suspicion) is based on neck ultrasound characteristics (described in section B2. Risk of malignancy in a thyroid nodule during childhood), history of radiation, and signs of a pre-disposition syndrome. If there is a significant increase in nodule size or the ultrasound characteristics change over time, repeated fine-needle biopsy (FNB) should be performed. **Analysis of the presence of other oncogenic drivers and gene fusions (e.g., RET/PTC and NTRK fusions) may be considered in Bethesda 3, 4, or 5 due to increasing awareness that these are also associated with the presence of papillary thyroid carcinoma (PTC). In case a BRAF V600E mutation is found, the risk of the thyroid nodule being malignant is high but needs to be confirmed, for example, by frozen section during thyroid surgery. ^Total thyroidectomy after proven presence of MTC. ^^Alternatively, FNB can be performed; in case of differentiated thyroid carcinoma (DTC), a total thyroidectomy should be performed. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. Credit: Lebbink, C. A., Links, T. P., Czarniecka, A., Dias, R. P., Elisei, R., Izatt, L., Krude, H., Lorenz, K., Luster, M., Newbold, K., Piccardo, A., Sobrinho-Simões, M., Takano, T., Paul van Trotsenburg, A. S., Verburg, F. A., & van Santen, H. M. (2022). 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. European Thyroid Journal, 11(6), e220146. Retrieved Aug 2, 2024, from https://doi.org/10.1530/ETJ-22-0146.
References:
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed February 25, 2025.
- Vergamini LB, Frazier AL, Abrantes FL, et al.: Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr 164 (6): 1481-5, 2014.
- Golpanian S, Perez EA, Tashiro J, et al.: Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int 32 (3): 201-8, 2016.
- Pole JD, Zuk AM, Wasserman JD: Diagnostic and Treatment Patterns Among Children, Adolescents, and Young Adults with Thyroid Cancer in Ontario: 1992-2010. Thyroid 27 (8): 1025-1033, 2017.
- Schmidt Jensen J, Grønhøj C, Mirian C, et al.: Incidence and Survival of Thyroid Cancer in Children, Adolescents, and Young Adults in Denmark: A Nationwide Study from 1980 to 2014. Thyroid 28 (9): 1128-1133, 2018.
- Dermody S, Walls A, Harley EH: Pediatric thyroid cancer: An update from the SEER database 2007-2012. Int J Pediatr Otorhinolaryngol 89: 121-6, 2016.
- Lebbink CA, Links TP, Czarniecka A, et al.: 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur Thyroid J 11 (6): , 2022.
- Christison-Lagay ER, Baertschiger RM, Dinauer C, et al.: Pediatric differentiated thyroid carcinoma: An update from the APSA Cancer Committee. J Pediatr Surg 55 (11): 2273-2283, 2020.



