Health Library
Childhood Ependymoma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
- General Information About Childhood Ependymoma
- Molecular Features of Childhood Ependymoma
- Histopathological Classification of Childhood Ependymal Tumors
- Stage Information for Childhood Ependymoma
- Treatment Option Overview for Childhood Ependymoma
- Treatment of Childhood Myxopapillary Ependymoma
- Treatment of Childhood Nonmyxopapillary Spinal Ependymoma
- Treatment of Childhood Intracranial Ependymoma
- Treatment of Recurrent Childhood Ependymoma
- Latest Updates to This Summary (01 / 06 / 2025)
- About This PDQ Summary
General Information About Childhood Ependymoma
Primary brain tumors, including ependymomas, are a diverse group of diseases that together constitute the most common solid tumor of childhood. Immunohistochemical analysis, cytogenetic and molecular genetic findings, and measures of mitotic activity are increasingly used in tumor diagnosis and classification. Brain tumors are classified according to histology, but tumor location, extent of spread, molecular features, and age are important factors that affect treatment and prognosis.
According to the 2021 revision to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS), ependymal tumors are classified into the following ten main subtypes based on anatomical site and histopathological and molecular features:[1,2,3]
- Supratentorial ependymoma.
- Supratentorial ependymoma, ZFTA fusion–positive (formerly called RELA fusion–positive).
- Supratentorial ependymoma, YAP1 fusion–positive.
- Posterior fossa ependymoma.
- Posterior fossa ependymoma, group PFA.
- Posterior fossa ependymoma, group PFB.
- Spinal ependymoma.
- Spinal ependymoma, MYCN-amplified.
- Myxopapillary ependymoma.
- Subependymoma (supratentorial, posterior fossa, and spinal locations).
The PDQ childhood brain tumor treatment summaries are organized primarily according to the WHO Classification of Tumors of the CNS.[1,3] For a description of the classification of nervous system tumors and a link to the corresponding treatment summary for each type of brain tumor, see Childhood Brain and Spinal Cord Tumors Summary Index.
Incidence
Childhood ependymoma comprises approximately 9% of all childhood brain and spinal cord tumors, representing about 200 cases per year in the United States.[4,5]
Anatomy
Ependymomas arise from ependymal cells that line the ventricles and passageways in the brain and the center of the spinal cord (see Figure 1). Ependymal cells produce cerebrospinal fluid (CSF). These tumors are classified as supratentorial, posterior fossa (infratentorial), or spinal. In children, 65% to 75% of ependymomas arise in the posterior fossa around the fourth ventricle.[6] Less commonly, ependymomas present in the supratentorial compartment. Spinal ependymomas are rare in childhood.
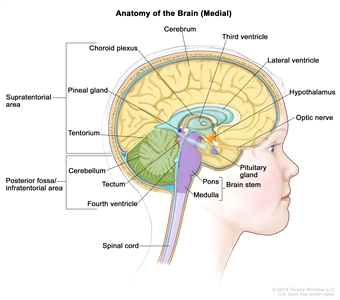
Figure 1. Anatomy of the inside of the brain, showing the pineal and pituitary glands, optic nerve, ventricles (with cerebrospinal fluid shown in blue), and other parts of the brain. The tentorium separates the cerebrum from the cerebellum. The infratentorium (posterior fossa) is the region below the tentorium that contains the brain stem, cerebellum, and fourth ventricle. The supratentorium is the region above the tentorium and denotes the region that contains the cerebrum.
Clinical Features
The clinical presentation of ependymoma is dependent on tumor location.
- Posterior fossa (infratentorial) ependymomas: Children with posterior fossa ependymomas may present with signs and symptoms of obstructive hydrocephalus caused by obstruction at the level of the fourth ventricle. They may also present with ataxia, neck pain, and/or cranial nerve palsies.
- Supratentorial ependymomas: Supratentorial ependymomas may result in headaches, seizures, or location-dependent focal neurological deficits.
- Spinal cord ependymomas: Spinal cord ependymomas, which are often the myxopapillary variant, tend to cause back pain, lower extremity weakness, and/or bowel and bladder dysfunction.
Diagnostic Evaluation
Every patient suspected of having an ependymoma is evaluated with diagnostic imaging of the whole brain and spinal cord. The most sensitive method available for evaluating spinal cord subarachnoid metastasis is spinal magnetic resonance imaging (MRI) performed with gadolinium. This is ideally done before surgery to avoid confusion with postoperative blood. If MRI is used, the entire spine is generally imaged in at least two planes with contiguous MRI slices performed after gadolinium enhancement.
If feasible, CSF cytological evaluation is conducted.[7] Despite the frequent finding of disseminated disease at the time of recurrence, metastatic disease at initial presentation is rare.[8][Level of evidence C2]
Prognostic Factors
Unfavorable factors affecting outcome (except as noted) include the following:
- Molecular characteristics.
Posterior fossa ependymomas are divided into the following two primary molecular groups on the basis of distinctive patterns of gene expression.[9,10,11,12]
- Posterior fossa A ependymoma (PF-EPN-A).
- PF-EPN-A occurs primarily in young children and is characterized by a largely balanced genomic profile, with an increased occurrence of chromosome 1q gain [13,14,15,16] and expression of genes and proteins previously shown to be associated with poor prognosis, such as tenascin C and epidermal growth factor receptor.[13,17,18]
- Gain of 1q confers a very poor prognosis despite complete resection and postoperative radiation therapy (5-year event-free survival rate, 81.5% for balanced 1q vs. 35.7% for gain 1q).[19][Level of evidence B4]
- A combined retrospective analysis of 663 patients from five nonoverlapping cohorts identified loss of 6q as a poor prognostic factor for patients with PF-EPN-A.[20] Loss of 6q was observed in 8.6% of PF-EPN-A cases, and it is more common in tumors with 1q gain. The subset of patients (n = 22) with both 1q gain and 6q loss had a particularly poor prognosis.
- A retrospective multi-institutional study compared patient-matched primary tumors with recurrent tumors. The study reported that the high-risk features of 1q gain and 6q loss were more frequent in recurrent tumors than in primary tumors, and these features remained associated with a poor prognosis.[21]
- Posterior fossa B ependymoma (PF-EPN-B).
- PF-EPN-B occurs primarily in older children and adults and is characterized by a more favorable prognosis and by numerous cytogenetic abnormalities involving whole chromosomes or chromosomal arms.[9,12,22]
- Patients with PF-EPN-B have a favorable outcome when compared with patients with PF-EPN-A. Patients with PF-EPN-B have a 5-year progression-free survival (PFS) rate of 73% and an overall survival (OS) rate exceeding 90%.[11,12]
- Gain of 1q is not a prognostic feature in patients with PF-EPN-B, whereas loss of chromosome 13q may confer a poor prognosis.[22]
Supratentorial ependymomas can be divided into the following two primary molecular groups on the basis of their gene fusion status:
- Supratentorial ependymoma, ZFTA fusion–positive (ST-EPN-ZFTA) (formerly termed RELA fusion–positive).
- While a retrospective analysis suggested that the RELA fusion predicted poorer prognosis,[11] subsequent reports suggest that patients with RELA fusions who undergo a complete resection and postoperative radiation have relatively favorable survival rates that are in the range of 80% at 5 years.[11,19,23,24] Retrospective studies suggest a poor outcome for patients who undergo complete surgical resections but do not receive postoperative radiation therapy.[11]
- Homozygous deletion of CDKN2A has been associated with a poor prognosis in patients with ST-EPN-ZFTA.[25][Level of evidence B4] CDKN2A deletion has also been reported as a secondary event in recurrent ependymoma.[26]
- Supratentorial ependymoma with YAP1 fusions (ST-EPN-YAP1).
Spinal ependymomas can be separated by methylome studies, but molecular classification does not provide any clinicopathological advantage over histopathological classification for myxopapillary ependymoma and subependymoma. However, molecular classification is useful for identifying spinal ependymoma with MYCN amplification, which has been associated with a poor prognosis. There is a paucity of data on the optimal risk stratification of spinal ependymoma in children, although inferring from adults, a complete resection confers a favorable prognosis.
- Spinal ependymoma, MYCN-amplified (SP-EPN-MYCN).
- Posterior fossa A ependymoma (PF-EPN-A).
- Younger age at diagnosis. Younger age at diagnosis has historically been a poor prognostic factor, although this could partially result from the common practice of avoiding or deferring radiation in children younger than 3 years. In a prospective Children's Oncology Group (COG) trial (ACNS0121 [NCT00027846]), immediate postoperative radiation therapy was given to all children older than 1 year after gross-total resection or near-total resection. The study demonstrated that there was no significant difference in 5-year PFS or OS between patients aged 1 to 3 years and patients aged 3 to 21 years.[19]
- Anaplastic histology. Anaplastic histology has been associated with a poor prognosis.[32][Level of evidence B4]; [33,34,35,36]; [37][Level of evidence C1]; [38][Level of evidence C2] However, the distinction between grade 2 and grade 3 disease has significant interobserver variability, confounding the use of anaplasia as a prognostic factor.[39] The 2021 WHO Classification of Tumors of the CNS no longer uses the term anaplastic ependymoma and allows only a histologically defined diagnosis of ependymoma in the integrated diagnosis. Within the layered report, a pathologist can still choose to assign either CNS WHO grade 2 or 3 to a tumor on the basis of its histological features.[2,3]
- Subtotal resection. Subtotal resection confers a very poor prognosis.[19,35,36]; [32][Level of evidence B4]
- Lower doses of radiation. Lower doses of radiation or chemotherapy-only protocols confer a poor prognosis.[12,23,40,41]
Follow-Up After Treatment
Surveillance neuroimaging, coupled with clinical assessments, is generally recommended after treatment for ependymoma. In a report of 198 patients with ependymoma, 90 experienced a relapse. Patients whose relapsed tumor was detected by routine surveillance imaging had superior second PFS than patients whose relapsed tumor was detected by clinical symptomology. The latter were more likely to have metastatic disease at relapse. It is not known whether these patients also had more biologically aggressive disease, although the median time to relapse and the median time from last surveillance imaging was the same in both groups.[42]
Most practitioners obtain MRI of the brain and/or spinal cord at the following intervals:[43][Level of evidence B4]
- First 2 to 3 years after treatment: Every 3 to 4 months.
- Four to 5 years after treatment: Every 6 months.
- More than 5 years after treatment: Annually because of the high incidence of late recurrences.
References:
- Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. IARC Press, 2016.
- Louis DN, Perry A, Wesseling P, et al.: The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23 (8): 1231-1251, 2021.
- WHO Classification of Tumours Editorial Board, ed.: WHO Classification of Tumours: Central Nervous System Tumours. Vol. 6. 5th ed. IARC Press; 2021.
- Gurney JG, Smith MA, Bunin GR: CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, Chapter 3, pp 51-63. Also available online. Last accessed February 9, 2024.
- Ostrom QT, Gittleman H, Truitt G, et al.: CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol 20 (suppl_4): iv1-iv86, 2018.
- Andreiuolo F, Puget S, Peyre M, et al.: Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol 12 (11): 1126-34, 2010.
- Moreno L, Pollack IF, Duffner PK, et al.: Utility of cerebrospinal fluid cytology in newly diagnosed childhood ependymoma. J Pediatr Hematol Oncol 32 (6): 515-8, 2010.
- Benesch M, Mynarek M, Witt H, et al.: Newly Diagnosed Metastatic Intracranial Ependymoma in Children: Frequency, Molecular Characteristics, Treatment, and Outcome in the Prospective HIT Series. Oncologist 24 (9): e921-e929, 2019.
- Wani K, Armstrong TS, Vera-Bolanos E, et al.: A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123 (5): 727-38, 2012.
- Witt H, Mack SC, Ryzhova M, et al.: Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20 (2): 143-57, 2011.
- Pajtler KW, Witt H, Sill M, et al.: Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 27 (5): 728-43, 2015.
- Ramaswamy V, Hielscher T, Mack SC, et al.: Therapeutic Impact of Cytoreductive Surgery and Irradiation of Posterior Fossa Ependymoma in the Molecular Era: A Retrospective Multicohort Analysis. J Clin Oncol 34 (21): 2468-77, 2016.
- Mendrzyk F, Korshunov A, Benner A, et al.: Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res 12 (7 Pt 1): 2070-9, 2006.
- Korshunov A, Witt H, Hielscher T, et al.: Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol 28 (19): 3182-90, 2010.
- Kilday JP, Mitra B, Domerg C, et al.: Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children's Cancer Leukaemia Group (CCLG), Societe Francaise d'Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin Cancer Res 18 (7): 2001-11, 2012.
- Godfraind C, Kaczmarska JM, Kocak M, et al.: Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol 124 (2): 247-57, 2012.
- Korshunov A, Golanov A, Timirgaz V: Immunohistochemical markers for intracranial ependymoma recurrence. An analysis of 88 cases. J Neurol Sci 177 (1): 72-82, 2000.
- Andreiuolo F, Le Teuff G, Bayar MA, et al.: Integrating Tenascin-C protein expression and 1q25 copy number status in pediatric intracranial ependymoma prognostication: A new model for risk stratification. PLoS One 12 (6): e0178351, 2017.
- Merchant TE, Bendel AE, Sabin ND, et al.: Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J Clin Oncol 37 (12): 974-983, 2019.
- Baroni LV, Sundaresan L, Heled A, et al.: Ultra high-risk PFA ependymoma is characterized by loss of chromosome 6q. Neuro Oncol 23 (8): 1360-1370, 2021.
- Donson AM, Bertrand KC, Riemondy KA, et al.: Significant increase of high-risk chromosome 1q gain and 6q loss at recurrence in posterior fossa group A ependymoma: A multicenter study. Neuro Oncol 25 (10): 1854-1867, 2023.
- Cavalli FMG, Hübner JM, Sharma T, et al.: Heterogeneity within the PF-EPN-B ependymoma subgroup. Acta Neuropathol 136 (2): 227-237, 2018.
- Upadhyaya SA, Robinson GW, Onar-Thomas A, et al.: Molecular grouping and outcomes of young children with newly diagnosed ependymoma treated on the multi-institutional SJYC07 trial. Neuro Oncol 21 (10): 1319-1330, 2019.
- Fukuoka K, Kanemura Y, Shofuda T, et al.: Significance of molecular classification of ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogeneous group of tumors. Acta Neuropathol Commun 6 (1): 134, 2018.
- Jünger ST, Andreiuolo F, Mynarek M, et al.: CDKN2A deletion in supratentorial ependymoma with RELA alteration indicates a dismal prognosis: a retrospective analysis of the HIT ependymoma trial cohort. Acta Neuropathol 140 (3): 405-407, 2020.
- Milde T, Pfister S, Korshunov A, et al.: Stepwise accumulation of distinct genomic aberrations in a patient with progressively metastasizing ependymoma. Genes Chromosomes Cancer 48 (3): 229-38, 2009.
- Andreiuolo F, Varlet P, Tauziède-Espariat A, et al.: Childhood supratentorial ependymomas with YAP1-MAMLD1 fusion: an entity with characteristic clinical, radiological, cytogenetic and histopathological features. Brain Pathol 29 (2): 205-216, 2019.
- Ghasemi DR, Sill M, Okonechnikov K, et al.: MYCN amplification drives an aggressive form of spinal ependymoma. Acta Neuropathol 138 (6): 1075-1089, 2019.
- Swanson AA, Raghunathan A, Jenkins RB, et al.: Spinal Cord Ependymomas With MYCN Amplification Show Aggressive Clinical Behavior. J Neuropathol Exp Neurol 78 (9): 791-797, 2019.
- Scheil S, Brüderlein S, Eicker M, et al.: Low frequency of chromosomal imbalances in anaplastic ependymomas as detected by comparative genomic hybridization. Brain Pathol 11 (2): 133-43, 2001.
- Raffeld M, Abdullaev Z, Pack SD, et al.: High level MYCN amplification and distinct methylation signature define an aggressive subtype of spinal cord ependymoma. Acta Neuropathol Commun 8 (1): 101, 2020.
- Massimino M, Miceli R, Giangaspero F, et al.: Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol 18 (10): 1451-60, 2016.
- Merchant TE, Jenkins JJ, Burger PC, et al.: Influence of tumor grade on time to progression after irradiation for localized ependymoma in children. Int J Radiat Oncol Biol Phys 53 (1): 52-7, 2002.
- Korshunov A, Golanov A, Sycheva R, et al.: The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer 100 (6): 1230-7, 2004.
- Tamburrini G, D'Ercole M, Pettorini BL, et al.: Survival following treatment for intracranial ependymoma: a review. Childs Nerv Syst 25 (10): 1303-12, 2009.
- Massimino M, Barretta F, Modena P, et al.: Second series by the Italian Association of Pediatric Hematology and Oncology of children and adolescents with intracranial ependymoma: an integrated molecular and clinical characterization with a long-term follow-up. Neuro Oncol 23 (5): 848-857, 2021.
- Amirian ES, Armstrong TS, Aldape KD, et al.: Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology 39 (2): 116-24, 2012.
- Tihan T, Zhou T, Holmes E, et al.: The prognostic value of histological grading of posterior fossa ependymomas in children: a Children's Oncology Group study and a review of prognostic factors. Mod Pathol 21 (2): 165-77, 2008.
- Ellison DW, Kocak M, Figarella-Branger D, et al.: Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed 10: 7, 2011.
- Vaidya K, Smee R, Williams JR: Prognostic factors and treatment options for paediatric ependymomas. J Clin Neurosci 19 (9): 1228-35, 2012.
- Zapotocky M, Beera K, Adamski J, et al.: Survival and functional outcomes of molecularly defined childhood posterior fossa ependymoma: Cure at a cost. Cancer 125 (11): 1867-1876, 2019.
- Klawinski D, Indelicato DJ, Hossain J, et al.: Surveillance imaging in pediatric ependymoma. Pediatr Blood Cancer 67 (11): e28622, 2020.
- Massimino M, Barretta F, Modena P, et al.: Pediatric intracranial ependymoma: correlating signs and symptoms at recurrence with outcome in the second prospective AIEOP protocol follow-up. J Neurooncol 140 (2): 457-465, 2018.



