As the prevalence of children with autism spectrum disorder (ASD) continues to rise in the United States, a handful of Autism Centers of Excellence (ACEs) across the country are spearheading efforts to increase our understanding of ASD and develop novel interventions. Wendy Chung, MD, PhD, Chief of Clinical Genetics at NewYork-Presbyterian Morgan Stanley Children’s Hospital, and Jeremy Veenstra-VanderWeele, MD, Director of Child and Adolescent Psychiatry at NewYork-Presbyterian Morgan Stanley Children’s Hospital, are recipients of one of the prestigious ACE grants from the National Institutes of Health (NIH). Their groundbreaking study entitled, the Prospective Genetic Risk Evaluation and Assessment (PROGRESS) in Autism Center, is a three-part project that combines genomics, developmental neuroscience, autism assessment and diagnosis, and psychosocial assessment.
This is a forward-looking project to understand how we approach genetic risk information about autism that is well prior to any behavioral or developmental indication.
— Dr. Jeremy Veenstra-VanderWeele
Genetic Testing
Although genetic testing is not yet routine for patients with autism, researchers are learning that the information gleaned can have profound implications on the timing of early intervention. The PROGRESS study is connected to a larger study led by Dr. Chung, where researchers are evaluating whole genome sequencing to improve newborn screening for rare genetic conditions, including ASD.
The Genomic Uniform-screening Against Rare Diseases In All Newborns (GUARDIAN) study will be conducted in approximately 100,000 screened infants and is available to all newborns at NewYork-Presbyterian/
“Ten years from now we will most likely use genetic screening at birth instead of the current metabolic screening. With all of that genetic information, we can potentially predict whether a baby will go on to develop autism based on a genetic condition,” says Dr. Veenstra-VanderWeele.
Groundbreaking Autism Research
In the PROGRESS study, Drs. Chung, Veenstra-VanderWeele, and colleagues will prospectively and longitudinally follow 240 out of 100,000 infants who have increased genetic risk for autism and compare them to 120 matched controls without autism risk. The three-part Project is supported by four Cores to advance our understanding of genetic risk, parental impact, and neurobehavioral trajectories that predict autism.
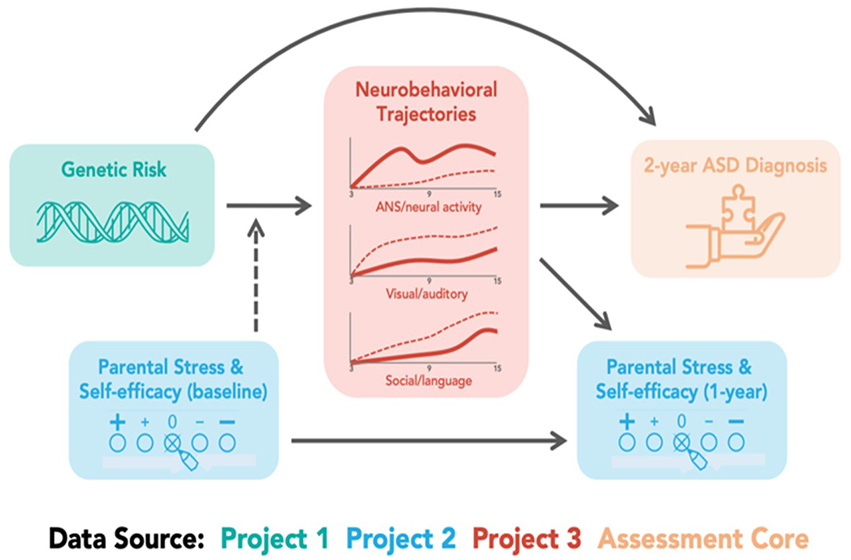
Figure 9. Data analyses span Projects and Cores to advances our understanding of genetic risk, parental impact, and neurobehavioral trajectories that predict autism.
“The goal is to think about 200 or so genes that we can point to as contributing to autism risk. Dr. Chung and Dr. Yufeng Shen, PhD, will be working to identify genes and improve prediction with genome sequencing instead of the standard metabolic screening,” says Dr. Veenstra-VanderWeele, who notes that infants who are identified with an autism gene variant will be followed over the first 2 years of life.
“This study is designed to leave no child behind as we strive to better understand early childhood brain development starting shortly after birth for neurogenetic conditions that can be associated with autism,” says Dr. Chung.
This study is designed to leave no child behind as we strive to better understand early childhood brain development starting shortly after birth for neurogenetic conditions that can be associated with autism.
— Dr. Wendy Chung
The researchers are also very interested in the impact on parents who receive information about genetic risk for autism, including the effects on parenting and self-efficacy. “We are working closely with parents to understand how they are coping and their emotional health,” says Dr. Veenstra-VanderWeele, who notes that Paul Appelbaum, MD and Matthew Lebowitz, PhD, are Lead and Co-Lead of Project 2, respectively.
Finally, the researchers are studying the impact of genomic risk variants on a child’s neurological, cognitive, and behavioral development, with the ultimate goal of recommending early intervention as soon as an autism diagnosis is confirmed. Dima Amso, PhD, and William Fifer, PhD, are Lead and Co-Lead of Project 3, respectively.
“At a few months of age, we will perform detailed developmental assessments—autonomic system function, brain waves with electroencephalogram, visual attention—and then follow their development across 6, 9, and 12 months, including how they are engaging with their parents,” says Dr. Veenstra-VanderWeele.
Supporting the three Projects in the PROGRESS in Autism study are an Administrative Core led by Drs. Chung and Veenstra-VanderWeele, a Statistical Core led by Melanie Wall, PhD, and Dr. Shen, an Assessment Core led by Stephen Kanne, PhD, and a Dissemination and Outreach Core led by Dr. Veenstra-VanderWeele and Gazi Azad, PhD.
A Unique Model of Care
NewYork-Presbyterian is recognized as a national and international leader in diagnosing and treating patients with ASD, largely owing to the Center for Autism and the Developing Brain (CADB), a collaborative program between NewYork-Presbyterian, Weill Cornell Medicine, and Columbia University Vagelos College of Physicians and Surgeons. The world-renowned CADB is particularly unique because it is equipped to assesses individuals from the toddler years all the way through adulthood. Directed by Dr. Kanne, CADB is located at NewYork-Presbyterian’s 214-acre Westchester Division campus in White Plains, New York. The CADB is an integral part of the PROGRESS in Autism study.
“If we are able to identify a child at birth who has an increased likelihood of developing autism, we could follow them and intervene as soon as it is clear that they have developmental delays or a trajectory that predicts autism,” says Dr. Veenstra-VanderWeele.




