Every year approximately 6,300 children are born with pulmonary valve disease, and, in most cases, their treatment will involve a valve replacement at some point during their lives. Despite the advancements in heart valve replacement over the past several decades, there has been remarkably little progress for pediatric patients, who need valve options that grow with them. Most mechanical valves are available only in adult sizes, and tissue-based valves from an animal or human can wear out quickly. As a result, children face the possibility of multiple, invasive surgeries before they reach adulthood.
Since 2021, Oliver Barry, MD, a pediatric interventional cardiologist at NewYork-Presbyterian and Columbia, has been involved in a first-in-human clinical trial for a novel pulmonary valve replacement known as the Autus Size-Adjustable Valve, which can be expanded using minimally invasive techniques. This new technology provides the promise of being the first treatment option that can grow with a child and improve quality of life for them.
Below, Dr. Barry discusses how the Autus Valve has been used to treat patients at NewYork-Presbyterian and how this innovative approach has the potential to transform the treatment of pediatric congenital heart disease.
What is the Autus Valve and how does it work?
The Autus Valve is an engineered technology that closely mimics the geometric design of the human venous valve, and it is made of a stainless-steel frame and leaflets that are made from a synthetic material with a long history of clinical use in children. Venous valve leaflets are tall, and when a vein is small in diameter, there’s a lot of valve tissue that overlaps to prevent leaks. As the vein grows wider, the venous valve leaflets still touch and still work, but their point of contact is less. The Autus Valve has similarly designed leaflets supported by the stainless-steel frame, and the frame can be expanded with a balloon to a larger diameter. The stent frame shortens in height as the valve gets wider, mimicking what a venous valve does naturally.
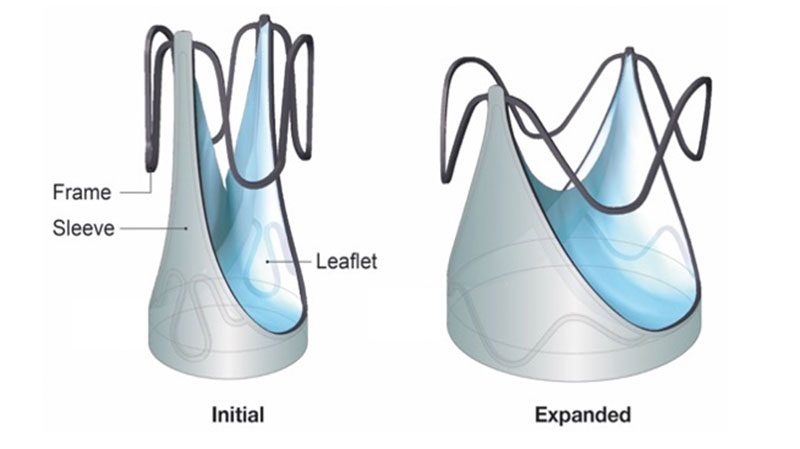
Autus Size-Adjustable Valve shown at initial and fully expanded sizes. Image courtesy of Autus Valve Technologies, Inc.
The Autus Valve can be balloon expanded before it’s implanted to match the size of each individual patient and is sewn in via open heart surgery just like any other replacement valve. As the child grows, the diameter of the valve can be expanded through minimally invasive transcatheter balloon dilations, in which we enter through a vein in the leg. The diameter can go from about 12 millimeters up to 22 millimeters, which would usually be an adequate size for a young adult, effectively allowing the valve to grow with them as they get older.
How is the Autus Valve trial designed?
Initially, this was an early feasibility trial – designed as a prospective, single-arm, multicenter study to evaluate the safety of the Autus Valve. The trial was initially approved for patients ages two to 10 years old, but the age range was extended to patients 18 months to 16 years who need a pulmonary valve replacement. The Autus Valve is now under investigation in an FDA-approved pivotal study, which is designed to evaluate safety and effectiveness of the device. The study will enroll approximately 50 patients and will include 10-15 U.S hospitals.
NewYork-Presbyterian and Columbia was only one of three sites participating when the early feasibility study started. How did you come to be involved?
When I was in my fellowship at Boston Children’s Hospital, I actually worked on the animal studies for the Autus Valve, so Dr. Sophie-Charlotte Hofferberth, the inventor of the Autus Valve, knew me well. The trial required each site to have two lead investigators – an interventional cardiologist like me and a cardiothoracic surgeon. My partner in the trial, Emile Bacha, MD, Chief of the Division of Cardiac, Thoracic, and Vascular Surgery at NewYork-Presbyterian and Columbia, is also well known in Boston from his previous work there. So when they were selecting sites, it was a combination of familiarity with us and also the reputation of NewYork-Presbyterian and Columbia as national leaders in both pediatric cardiac surgery and interventional cardiology.
The expertise and resources we have at NewYork-Presbyterian and Columbia enable us to engage in incredibly innovative and groundbreaking research and provide the highest quality of care to our patients, which made our site a strong fit for trialing this new technology. NewYork-Presbyterian’s Pediatric Heart Center is one of the largest and most well-known pediatric cardiology and cardiac surgery centers in the United States.
We also have one of the largest volume congenital catheterization labs in the country. We do somewhere around 1,200 cases a year and handle a lot of complex procedures – there are no procedures we don’t do. One of our responsibilities as a leading center for congenital heart disease is to push boundaries and pursue innovations for not only our patients, but patients around the country and world.
How many patients at NewYork-Presbyterian have received the Autus Valve so far? And what’s an example of how it has been used?
We have implanted the Autus Valve in four patients so far at NewYork-Presbyterian and have another two that we are currently assessing. What we look for is a child that might meet any indication for a pulmonary valve replacement. That includes right-sided congenital heart diseases like double outlet right ventricle, pulmonary atresia with intact ventricular septum, pulmonary stenosis, and Tetralogy of Fallot (TOF).
One of the earliest opportunities we had to implant the Autus Valve was in a TOF patient named Yasin Samad. He was diagnosed shortly after birth with TOF by Maria Thanjan, MD, FAAP, Chief of Pediatric Cardiology at NewYork-Presbyterian Queens, who referred him to Dr. Bacha when he required surgery at 6 months old. Dr. Bacha performed open heart surgery on him then and again at 16 months to help improve blood flow to his lungs, which he was able to do without replacing the valve. Generally, in patients with TOF, the valve doesn’t wear out until their teenage years. But when Yasin was 7, he started showing symptoms of valve failure.
Recognizing that options were limited, Dr. Bacha brought me in to review Yasin’s case and eligibility for the Autus Valve. We determined that Yasin would be an ideal candidate, presented his case to the Autus Valve trial investigator group, and then enrolled him in the trial. Yasin was the second patient in our center to receive this valve and the fifth in the country.
When the time comes that Yasin outgrows his Autus Valve completely, we anticipate using this existing valve as a landing zone for a new tissue valve to effectively replace it without having to remove it. However, this is still a concept and has not been evaluated clinically.
How do you think this technology will change the future treatment of congenital heart defects?
The Autus Valve technology has the potential to be completely disruptive to the field. Dealing with pulmonary valve disease in pediatrics is a very difficult problem surgically and in the catheterization lab. So a valve that works and continues to work at many different sizes is an idea that will ideally be replicated, improved, evolved, and translated to the different positions of the heart.
This kind of innovation and research paves the way for us to be able to help more patients and make these novel devices more widely available. I think this is going to be the first of many technologies like this that will change the game for kids with congenital heart disease and especially valve disease.
Note: The Autus Size-Adjustable Valve is an investigational device. Limited by Federal (United States) Law to investigation use.




