Wound complications following total joint arthroplasties are uncommon but can put patients at substantially higher risk for periprosthetic joint infections – a difficult and costly complication to treat. As specialists in hip and knee reconstructive surgeries, Carl L. Herndon, MD, and H. John Cooper, MD, orthopedic surgeons at NewYork-Presbyterian and Columbia, are acutely aware that multiple factors that can influence incisional healing, down to what kind of dressing is used on wounds.
In a new paper published in a leading scientific journal, the Journal of Arthroplasty, Dr. Cooper, Dr. Herndon, and their colleagues at NewYork-Presbyterian and Columbia, including research associate and lead author Catelyn Woelfle, published the results of a study evaluating how two different wound closure approaches contribute to minimizing complications and facilitating healing. Below, Dr. Cooper and Dr. Herndon discuss the findings from their study and how such a seemingly small decision can lead to differentials in outcomes.
Study Background
For many years, we have used one design of mesh dressing with a 2-octyl cyanoacrylate adhesive for wound closure with excellent results. Recently, however, our team was introduced to a new design of the same type of dressing. The goal of both dressings is to reduce tension across the wound and expedite a new tissue connection; however we were unsure if small design differences between the two dressings would influence their clinical function.
Based on many previously published studies, 2-octyl-cyanoacrylate topical skin adhesives have shown promising results in joint arthroplasty patients, particularly for total knee and total hip, in which incisions often involve greater tension than incisions elsewhere on the body.
After we began using the newer dressing design, our group of arthroplasty surgeons collectively began to observe some complications. This prompted us to conduct this study to identify and elucidate the differences and the impact on patient outcomes. Determining the factors involved would not only address the potential for complications within our practice but could also serve to shape best practices for colleagues at other institutions.
Study Methods
The two surgical dressings appear quite similar. However, a closer look revealed subtle variations in design and delivery, which had not previously been studied. Our investigation sought to distinguish the differences between the two skin closure systems and determine the incidence of wound complications for each.
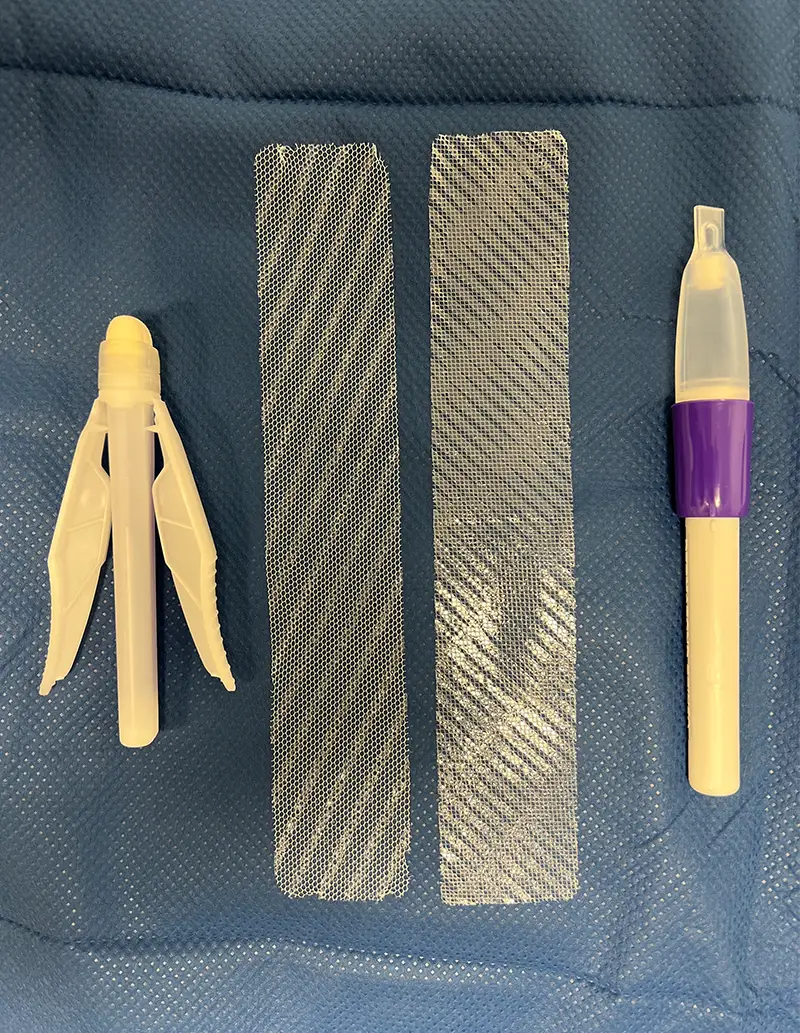
Comparison of (left) Mesh B, where the accelerant is in the applicator, versus (right) Mesh A, where the accelerant is on the mesh itself.
To this end, we conducted a retrospective cohort study of 212 primary total knee and 207 total hip arthroplasty cases performed by four surgeons here at NewYork-Presbyterian and Columbia from July 2023, to December 2023. All patients received the same postoperative care instructions regarding wound care and antibiotic prophylaxis. Both dressings had a 2-octyl-cyanoacrylate liquid adhesive formula applied topically to a polyester-based mesh overlaying the wound. Our original style of dressing – Mesh A, used in 274 cases – includes an accelerant embedded into the mesh to hasten polymerization with the glue to the top of the patch. The newer system – Mesh B, used in 145 cases – includes a similar accelerant, but it is contained within the adhesive applicator.
Key Findings
We hypothesized that polymerization occurs prematurely when the accelerant is contained within the adhesive applicator – before it is even applied to the mesh. As a result, the seal may not be as strong as when the adhesive is already present within the mesh. And, in fact, our clinical observations supported this hypothesis that there was often an incomplete seal of the incision with Mesh B.
Specifically, we found that:
- The overall wound complication rate was 4.8%, which is analogous to published studies on liquid adhesive dressings with mesh patches in total joint arthroplasty
- Certain complications were experienced at a significantly lower rate in Mesh A versus Mesh B, including wound complications (3.2% vs 7.6%), early periprosthetic joint infections (0.0% vs 2.8%), and 90-day reoperations for wound complications (0.4% vs 3.4%)
- There was little difference in wound healing outcomes between patients undergoing hip replacement and those undergoing knee replacement
Lessons Learned
By formally testing our observations, we were able to identify subtle differences in design that could potentially have major consequences for patients. While they may appear alike on the surface, these products are not necessarily interchangeable, and outcomes may be quite different. Based on the results of this study, we have discontinued the use of the Mesh B product going forward.
Our study demonstrated that as orthopedic surgeons, we need to be aware that each type of mesh dressing design has its pros and cons, just the way different types of implants and sutures do. It’s important to critically evaluate every new approach, protocol, or product before embracing the change wholeheartedly.





