Retinitis pigmentosa (RP) is a devastating inherited disease that induces progressive retinal degeneration causing blindness in some 1.5 million people worldwide. Finding a cure is no easy task as the condition’s origin can involve one or more of at least 3,100 mutations in over 80 different genes. Stephen H. Tsang, MD, PhD, an ophthalmic geneticist at NewYork-Presbyterian and Columbia’s Jonas Children's Vision Care specializing in retinal degeneration, is exploring the use of therapeutic genome surgery to preserve function in divergent forms of RP.
“Although gene therapy has shown promise in retinitis pigmentosa, it is complicated by the fact that defects in so many genes have been linked to the disorder and each genetic defect would require a different FDA Investigational New Drug Application (IND),” says Dr. Tsang. “While the mechanisms that cause certain mutations to lead to cell death in patients with retinitis pigmentosa are not fully understood, numerous studies suggest that metabolic aberrations may underlie or contribute to retinal degeneration. We believe the recent FDA approved gene editing tool CRISPR offers the best hope for restoring retinal function regardless of an individual’s genetic profile.”
Using CRISPR to Reprogram the Metabolome
Dr. Tsang is known worldwide for his pivotal research in reprogramming the metabolome as a therapeutic avenue for neurodegenerative disorders. He has been culturing stem cells since 1992 and, in 1995, he created the first mouse model for a recessive form of RP by applying genome engineering to stem cell technology. In his genome engineering laboratory at Columbia, Dr. Tsang applies his expertise to designing strategies in pre-clinical models with a goal toward developing precision therapies for patients.
“We are tackling RP and other neurodegenerative disorders in patient-specific engineered mouse models,” says Dr. Tsang. “In particular, our research team is focused on CRISPR approaches to reprogramming the glycolytic metabolome in the retina to promote cell survival. CRISPR is a simple, versatile, and precise method of genome engineering. Our hypothesis is that this method could be broadly applicable to retinal degenerative diseases regardless of the mutation.”
Using CRISPR, Dr. Tsang and his scientific colleagues seek to design a genome surgery with the potential to treat RP patients regardless of the underlying genetic defect. “A universal precision metabolome rejuvenation would be a vast improvement over the limited options now available to most RP patients, which do little to prevent blindness,” says Dr. Tsang. “Although each of the nearly 80 genes provokes RP in different ways, blindness usually occurs because the light sensing neurons experience anabolic biosynthetic failure and become deprived of energy.
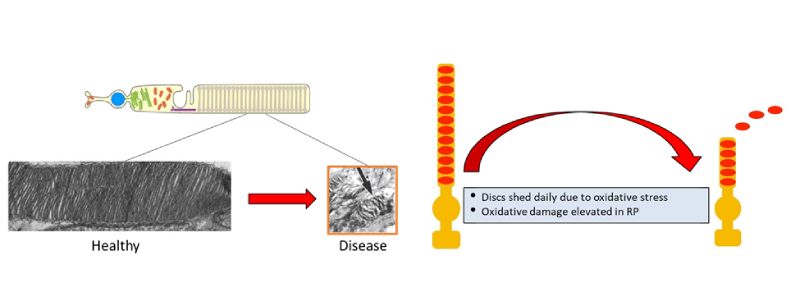
Rod outer segments shorten due to elevated oxidative stress & low anabolism.
Rods obtain energy by breaking down glucose sugar-lysis (i.e., glycolysis) to generate adenosine triphosphate (ATP), an energy supplying molecule, through glycolysis. Reduction in ATP energy production appears in both diseased and aging rods. This, in turn, leads to cone death and the loss of day vision. Dr. Tsang’s research team hypothesized that rejuvenating the glycolytic metabolome specifically within rods could thwart deterioration of the rods and cones thereby decelerating aging of the retina and preserving vision. Their proposed therapy calls on CRISPR genome surgery to edit the gene PHD (prolyl hydroxylase), which could rejuvenate rod glycolysis. During World War II, Otto Warburg, MD, observed a preference for anabolism in retinal cells, fetal cells, and rapidly proliferating tumor cells.
Dr. Tsang and his colleagues recently conducted a study using CRISPR-based gene therapy in both dominant and recessive preclinical models to determine if it can curtail RP by ablating PHD to rescue retinal degeneration. To achieve this outcome, they created a novel precision reprogramming method by bundling the components of the CRISPR genome surgery system into adeno-associated viral vectors (AAV). When injected into the retina, AAV expressed their genetic payload specifically only in rod photoreceptor neurons.
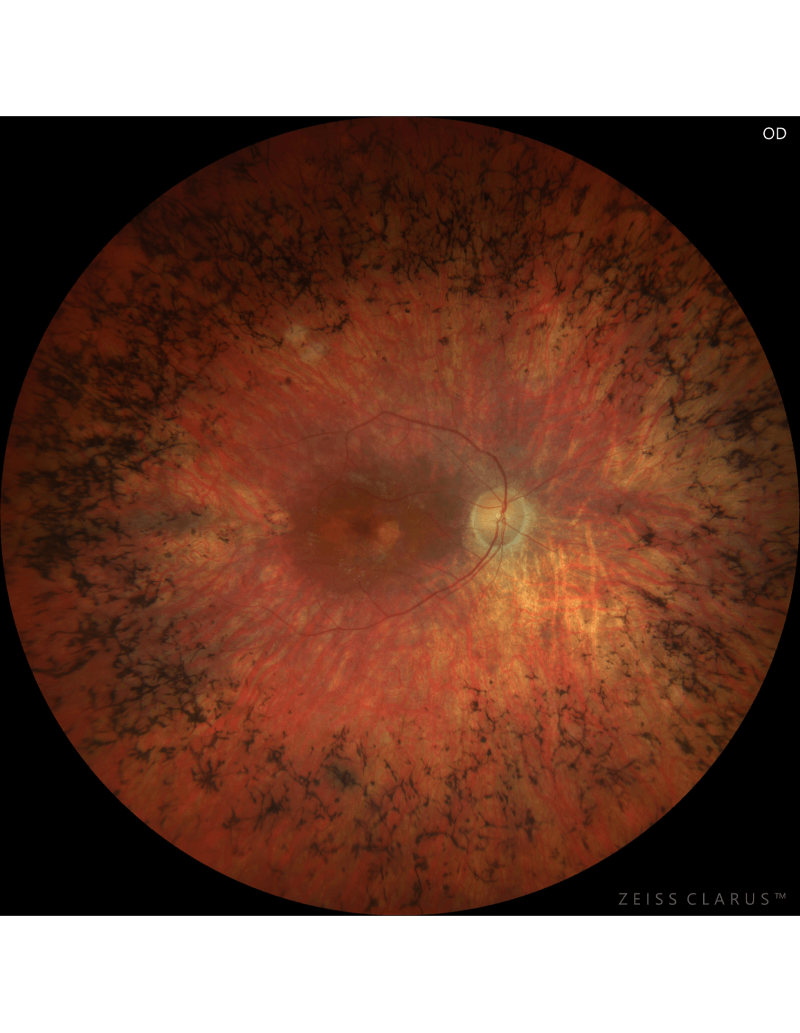
RP patient blinded by the same mutant rhodopsin gene as CRISPR treated mice
The researchers’ findings, published in Cell Reports Medicine, demonstrated that:
- CRISPR-based gene therapy can delay the progression of RP for about one month in mouse in mouse models of recessive and dominant forms of the disease (approximately 10 years in humans)
- Genetic ablation of PHD reprogrammed retinal metabolism
- PHD antagonists led to the sustained survival of both diseased rods and cones in both preclinical autosomal-recessive and dominant RP models
- Adeno-associated virus-mediated CRISPR-based therapeutic reprogramming of the aerobic glycolysis node may serve as a gene-agnostic treatment for patients with genetically heterogeneous RP caused by various genes.
“Overall, the impact of our data provides evidence that reprogramming metabolism can play an important role in treatment of neurodegenerations,” says Dr. Tsang. “Because CRISPR surgery specifically targets mutant photoreceptors in retinitis pigmentosa, with further research this cell-specific strategy may well provide a safer and effective treatment for retinal disorders caused by a diverse array of genetic deficiencies.”
According to Dr. Tsang, metabolic reprogramming using a non-gene-specific approach may help mitigate retinal degeneration. “This is an exciting time for implementation of precision medicine in ophthalmology,” says Dr. Tsang. “When I was a VP&S student, I thought I'd be just treating mice during my career as an attending physician. Our recent FDA meeting indicated that metabolome reprogramming is acceptable to the agency and findings in the Cell Reports Medicine. paved the way for a more widely applicable therapeutic strategy for patients with RP metabolome could be a promising approach for developing treatments to address any mutations the patient may have.”