NewYork-Presbyterian has established a unique, multidisciplinary collaboration to treating movement disorders through two dedicated academic centers:
- The Parkinson’s Disease and Movement Disorders Institute at NewYork-Presbyterian/Weill Cornell Medicine
- The Center for Parkinson’s Disease and Other Movement Disorders at NewYork-Presbyterian/Columbia
Two key contributors powering the success are Michael Kaplitt, MD, PhD, Executive Vice Chair of the Department of Neurological Surgery at NewYork-Presbyterian/Weill Cornell Medicine, and Gordon Baltuch, MD, PhD, Co-Chief of the Division of Functional Neurosurgery at NewYork-Presbyterian/Columbia. Dr. Kaplitt is a pioneer in the development of neurological gene therapy for Parkinson’s disease and performed the first treatment in a human patient 20 years ago. He has served as a lead investigator on many seminal gene therapy studies utilizing adeno-associated virus (AAV), an inert virus used to deliver gene therapy directly to the brain. Dr. Baltuch was one of the first neurosurgeons in the country to use deep brain stimulation (DBS) to reduce tremor and other symptoms in people with Parkinson’s disease and is one of the most experienced neurosurgeons in functional neurosurgery.
Together, Drs. Kaplitt and Baltuch are now combining their individual expertise to collaborate on a new randomized study of an innovative gene therapy – AAV-glutamic acid decarboxylase (AAV-GAD) – that holds promise for controlling Parkinson’s disease symptoms in people whose motor function remains impaired despite medication.
Delivering Gene Therapy Directly to the Subthalamic Nuclei
In this study, participants will be assigned to one of three groups for the duration of the study – AAV-GAD dose 1, AAV-GAD dose 2, and sham treatment. The treatments will be delivered to the subthalamic nuclei (STN), a key brain region involved in motor function. This is the location where DBS occurs in patients with Parkinson’s who become resistant to medical therapies or develop disabling side effects. This gene therapy offers a possible alternative to DBS, and in this trial, AAV-GAD is delivered into the STN via catheter infusion over a 2.5-3.5-hour period.
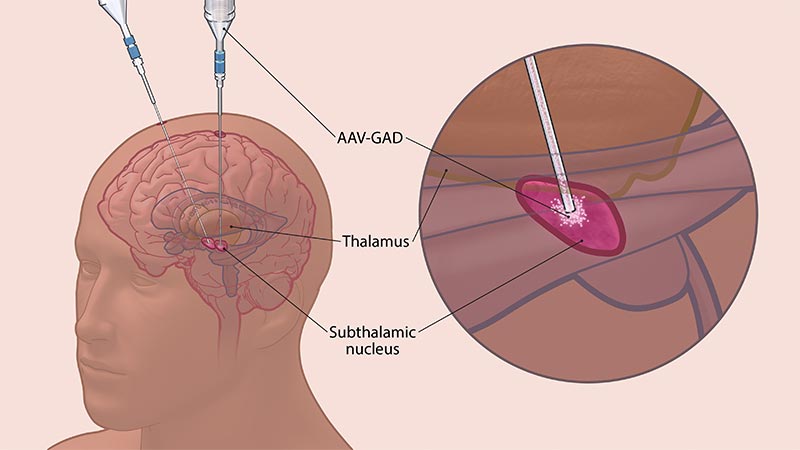
AAV-GAD is delivered directly to the subthalamic nucleus via catheter infusion.
People with Parkinson’s disease experience substantial reductions in the activity and amount of GABA — a major inhibitory neurotransmitter that helps quiet excessive neuronal firing in their brains — resulting in dysfunction in the brain circuitry responsible for coordinated movements. AAV-GAD is an investigational gene therapy designed to deliver the GAD gene to the STN to increase GABA production.
Because GAD is the rate-limiting enzyme in GABA synthesis, increasing STN GAD expression through gene therapy may result in the normalization of motor circuits and improve Parkinson’s disease symptoms without causing the kinds of side effects associated with existing therapies.
“Instead of implanting brain-stimulating electrodes attached to batteries that leave hardware in the body and then have to be managed, the idea is that we could put in a gene to change the way cells work and improve the function of the brain circuitry,” says Dr. Kaplitt.
We could put in a gene to change the way cells work and improve the function of the brain circuitry.
— Dr. Michael Kaplitt
Dr. Kaplitt, Dr. Baltuch, and the other investigators will monitor safety and tolerability of AAV-GAD delivered to the STN in patients with Parkinson’s throughout the study duration by tracking the number of participants that experience adverse events. They will also measure the effect of AAV-GAD on the Unified Parkinson’s Disease Study Rating Scale (MDS-UPDRS) Part 3 (motor function) score, tracking the mean change from baseline to weeks 12 and 26 for the AAV-GAD groups compared to the sham group.
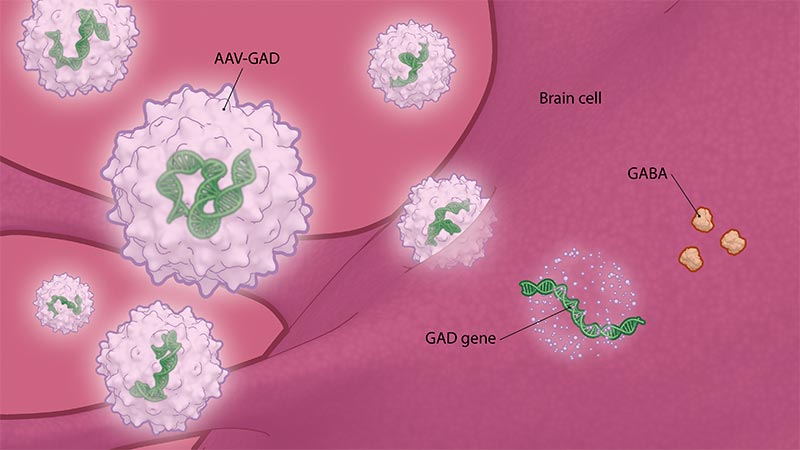
AAV-GAD is an investigational gene therapy designed to deliver the GAD gene to the STN to increase GABA production.
Building on a Legacy of Innovation
This new AAV-GAD trial builds off the milestones achieved in past ones led by Dr. Kaplitt including:
- A phase 1 study evaluating AAV-GAD in 12 patients who received the treatment on one side of the brain. No adverse events were reported, and patients experienced significant improvements in unified Parkinson’s disease rating scale (UPDRS) motor scores. The study represented the first time that gene therapy was evaluated in the human brain in a clinical trial. It was published as a cover article in Lancet in 2007.
- A phase 2 study assessing AAV-GAD in a randomized study where the treatment was administered to both sides of the brain in 45 patients at seven U.S. centers. Half received gene therapy and half had sham surgery. The differences between the two groups were significant: Half of the patients receiving gene therapy achieved dramatic improvements in their symptoms — including tremor, rigidity, and difficulty initiating movement — versus 14% of the control group. Patients receiving gene therapy reported a 23.1% improvement in motor scores, compared with a 12.7% improvement in the control group. The most common adverse events, headache and nausea, were likely related to surgery and resolved. The findings were published in Lancet Neurology in 2011.
The gene therapy is representative of what we are doing here and who we are. It is the kind of cutting-edge care that patients can get at a place like NewYork-Presbyterian.
— Dr. Gordon Baltuch
Since then, several long-term follow-up studies have been conducted, including those with imaging components. They showed that the gene therapy resulted in changes in brain network activity, including improvements in the way the brain processes and sorts information. Brain-sorting function becomes abnormal in Parkinson’s disease and was brought closer to normal in people who received gene therapy.
Movement disorders specialists at NewYork-Presbyterian have a history of developing and evaluating the latest treatments. They were among the first to offer DBS as well as high-intensity focused ultrasound, which has become a standard of care for tremors.
“This is the kind of work that can happen at an academic medical center like ours, where we work together across campuses to bring patients the most advanced treatments. We know this gene therapy works. We’re doing what we need to do to prove this and hope it will eventually become a standard of care,” says Dr. Baltuch. “The gene therapy is representative of what we are doing here and who we are. It is the kind of cutting-edge care that patients can get at a place like NewYork-Presbyterian.”





