NewYork-Presbyterian and Weill Cornell Medicine neurologist Samuel Bruce, MD, is conducting novel research to better understand cerebral amyloid angiopathy (CAA) and the related CAA-related inflammation (CAA-ri). CAA is a relatively common cerebrovascular disease in older individuals that can weaken blood vessels in the brain over time, raising the risk of hemorrhage and causing cognitive deficits. Less often, patients may develop CAA-ri, which is characterized by massive inflammation around the cerebral blood vessels, rapid decline in cognition, and fluid accumulation in the brain that can be visualized on magnetic resonance imaging.
Dr. Bruce is leading one of the first research studies to identify biomarkers differentiating patients with CAA from those with CAA-ri by comparing measures of blood-brain barrier (BBB) disruption and inflammation between the two groups of patients. "Little is known about why CAA-ri occurs and which patients with CAA are at risk of developing it," he explains. "I'm interested in learning what part inflammation plays in the CAA disease process, as well as figuring out what makes somebody more likely to develop CAA-ri rather than just CAA."
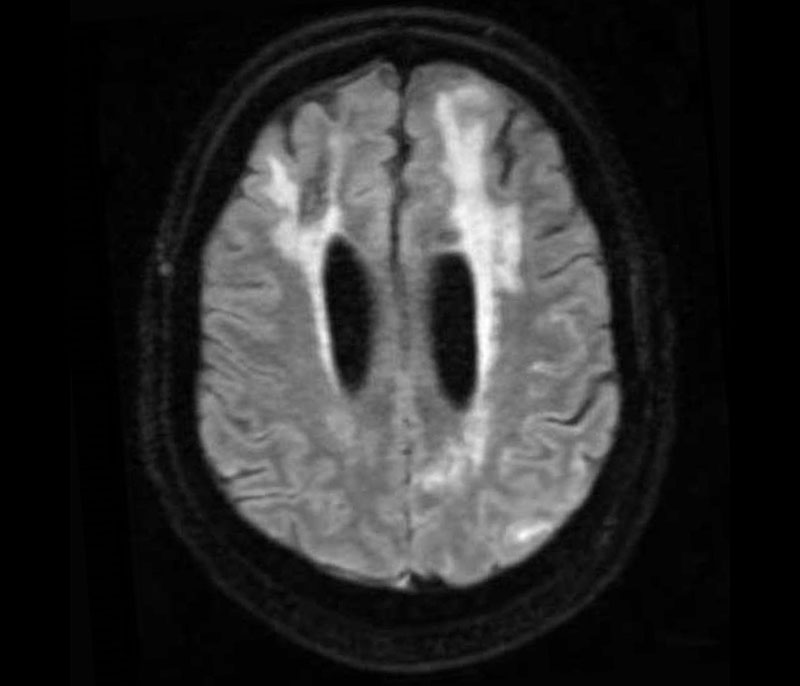
Image from a fluid attenuated inversion recovery (FLAIR) sequence from an MRI showing the imaging signature of CAA-ri: asymmetric cortical and subcortical hyperintensities suggestive of vasogenic edema and sulcal effusion.
He also hopes that the results of this work will help physicians better understand amyloid-related imaging abnormalities with edema and sulcal effusion (ARIA-E), a complication experienced by some patients receiving the newly FDA-approved immunotherapies for Alzheimer's disease. ARIA-E and CAA-ri look very similar on MRI and may be related.
A Tale of Two Pathologies
CAA most often occurs in people over age 50. Amyloid deposits build up in the walls of cerebral blood vessels. Over time, the walls become very fragile and may bleed. Amyloid deposits in brain tissue itself is a hallmark of Alzheimer's disease. "Alzheimer's and CAA are pretty closely linked," Dr. Bruce notes. "Having one doesn't necessarily guarantee you have the other, but many people who have one do have the other. Both conditions are associated with dementia and significant cognitive impairment."
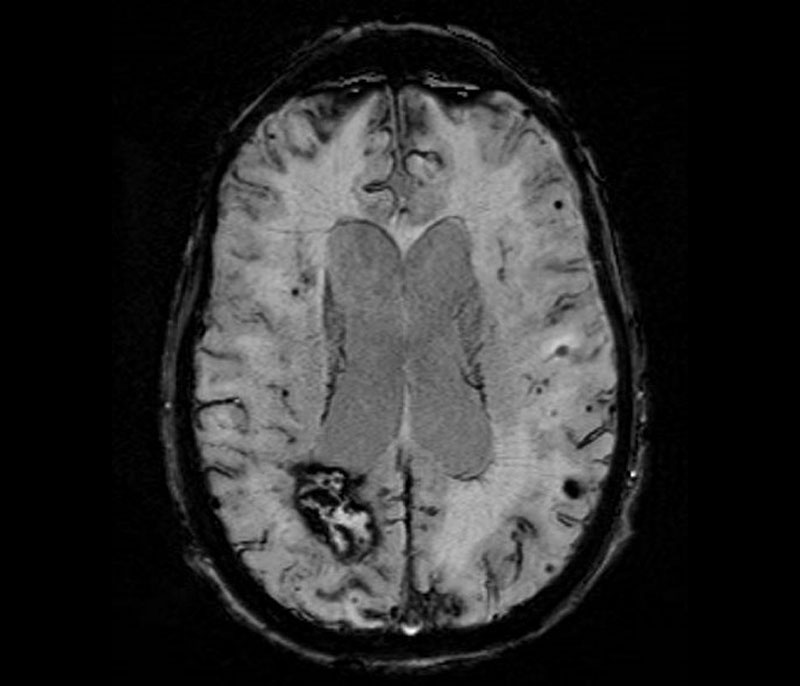
Susceptibility weighted imaging sequence of an MRI showing multiple imaging hallmarks of CAA: lobar intracerebral hemorrhage, multiple lobar microhemorrhages, and cortical superficial siderosis.
In contrast, CAA-ri is diagnosed more often in people age 40 and older. It is an autoimmune response to the amyloid deposits accumulating in arterial walls. Patients may experience headaches, seizures, debilitating focal deficits, and cognitive decline.
CAA raises the risk of intracerebral hemorrhage and can lead to subarachnoid hemorrhage. "It is mostly a hemorrhagic disease, but it's not solely a hemorrhagic disease," Dr. Bruce says. "When neurologists are training, there's a lot of emphasis on CAA as a bleeding disease, but it is so much more. It's really more of a chronic progressive disease of dementia and cognitive impairment."
He and his colleagues have also been studying the association between CAA and subdural hemorrhage. Indeed, they published a case report in the Annals of Internal Medicine Clinical Cases in January 2024 describing a 77-year-old woman with headache and confusion who was diagnosed with CAA-ri presenting as an acute nontraumatic subdural hemorrhage.
There are no approved treatments for CAA. People with general CAA typically receive palliative care and symptom management and are prepared for the possibility of dementia and cognitive impairment in the future. "We don't really have any strategies for slowing the progression of the disease. The more I read about Alzheimer's disease, the more I think that a lot of the lessons that have been learned about it over the last 30 years could very much apply to CAA," states Dr. Bruce.
On the other hand, CAA-ri can be treated with immunosuppressive therapies to reduce inflammation. "One of the reasons we are very interested in CAA-ri is that there is something we can do about it," he added.
A Transformative Study
In CAA-ri, the BBB is very obviously disrupted on MRI, as evidenced by the fluid development around it. In the new National Institutes of Health-funded study he is leading, Dr. Bruce aims to determine if markers of BBB disruption and inflammation differ between people with CAA and CAA-ri. To his knowledge, there is no other study of its kind being conducted.
Participants will undergo dynamic contrast enhanced MRI and will also have lumbar punctures to draw cerebrospinal fluid for analysis of possible biomarkers. These could include biomarkers of CAA or CAA-ri and/or indicators of disease progression. Dr. Bruce plans to recruit 10 patients with CAA and 10 with CAA-ri. If one or more biomarkers are identified, they would then be validated in a larger multicenter study.
A Hub for Neuroscience Leadership
Dr. Bruce notes that the expertise of NewYork-Presbyterian and Weill Cornell Medicine's neuroscience teams makes this kind of work possible. "CAA and CAA-ri are not well-studied conditions, and we see a decent number of patients here at NewYork-Presbyterian, probably more than a lot of other centers do," he asserted. "There is a collaborative culture here as well as tremendous resources and support that set up young investigators for success."




