Part of the mission of Och Spine at NewYork-Presbyterian is to pursue innovation that can impact the future of spine surgery. With that goal in mind, every three years an outstanding junior faculty member is appointed as the Leonard and Fleur Harlan Clinical Scholar to advance research projects in the field of neurosurgery. The latest physician to receive the award is Ibrahim Hussain, MD, a neurological spine surgeon at Och Spine at NewYork-Presbyterian and Weill Cornell Medicine, who will use the funding to advance his next-generation research in biologic disc replacement and stem-cell based therapies for disc regeneration and repair.
Dr. Hussain is involved in the development of state-of-the-art intraoperative technologies to make spine surgery safer, less invasive, and more precise, including 3D computer-assisted navigation, endoscopy, and augmented reality. In 2022, he achieved a spine surgery milestone when he performed the first 3D navigation-guided endoscopic transforaminal lumbar interbody fusion in New York City. Below, Dr. Hussain discusses his research and how this award will help improve care for patients who undergo spine surgery.
Can you tell us more about the research that the Leonard and Fleur Harlan Clinical Scholar award will help fund?
I have been collaborating with my mentor, Roger Härtl, MD, co-director of Och Spine at NewYork-Presbyterian and Weill Cornell Medicine; Lawrence Bonassar, PhD, the Daljit S. and Elaine Sarkaria Professor at Cornell University’s Meinig School of Biomedical Engineering; and other researchers from Cornell University on the development of biologic strategies to treat diseased spinal discs. All of this research will continue to be supported by the fund, which also includes research on biologic disc replacement and high-density collagen gels to seal annular defects. The aim is to improve outcomes for herniated disc surgery and reduce the need for revision procedures.
Our initial goals are focused on developing bench-based basic science projects to help us understand how cells interact with each other, and to create life-like biological disc replacements and scaffolding. Once we have achieved certain metrics in this arena, we will transition to animal studies, first in small mammals (mice, rats) and then in large mammals (sheep, goats, pigs) who more accurately replicate the size and biomechanical stresses of the human spine.
My hope is that with these innovations, we can halt the “degeneration cascade” that often begins in young, healthy individuals who are at greater risk for long-term implications if they undergo spine surgery, and also to provide alternative solutions to fusion operations in others who are already far into the degenerative disc process.

Dr. Ibrahim Hussain, Neurological Surgeon at Och spine at Newyork-Presbyterian and assistant professor of neurological surgery at Weill Cornell Medicine
What is novel about your approach to treating disc regeneration and repair?
Our current work with regeneration and repair is unique because while the process of mitochondrial transfer (MT) has been described in other musculoskeletal regions, it has never been described in the intervertebral discs We hypothesized that it is through various mechanisms of MT that mesenchymal stem cells, which are multipotent stem cells found in bone marrow, help repair cells in the degenerating disc, specifically annulus fibrosis cells in the covering of the disc.
MT transfer represents a novel avenue for enhancing cellular therapies that will be the main target of this seed proposal. We plan to investigate the potential of patient-derived MSCs to donate MT to injured AF cells and tissue; strategies to enhance MSCs’ capacity to donate healthy MT to AF cells; and develop new materials to enhance donation by MSCs. All these strategies will be employed to enhance IVD repair in vivo and collectively, will be the first studies to harness the potential of MSC MT donation to enhance healing of degenerated IVD.
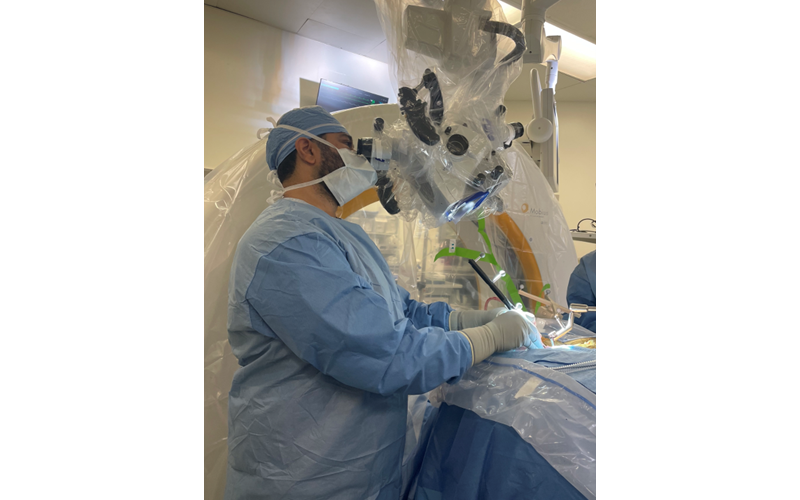
Dr. Hussain performing a minimally invasive cervical spine decompression procedure
What have you learned so far?
This has been a very exciting area, especially given the rapid expansion of artificial device-based disc replacements over the past decade and the growing need to provide an alternative to traditional fusion surgeries. Relative to our research, we have learned that biologic artificial discs need to be placed in a specific biodegradable scaffolding and that different stressors may be involved in how well the biologic disc will incorporate into the host body.
Our biggest challenge right now is being able to translate the findings we see in the lab into large animal models, though we have already demonstrated short-term positive outcomes in sheep and pigs. Looking even further down the road, getting these types of therapies to be reviewed by the FDA requires an enormous financial and time commitment due to the rigorous regulatory process. Cell-based therapies have a particularly challenging time progressing through the FDA clinical trial phases because of their potential unknown long-term implications. However, I am optimistic that we will be able to overcome this challenge in the next 10 years.
How does your research impact your approach to patient care?
My approach has definitely changed with the more I learn, as working on these studies allows me to give more granular information to my patients so they can make informed decisions about whether or not to proceed with surgery. It also gives me an opportunity to discuss how they can potentially contribute to the future of neurosurgery and spine surgery by allowing me to use samples from their surgery in our lab for research purposes. My priority always remains delivering safe, effective, and empathetic care to my patients, both in and out of the operating room.
What motivates you to continue this work in spine surgery?
Innovation is my driving force and most of the emphasis on the treatment for disc degeneration and herniations has revolved around surgical advancements, such as the development of minimally invasive and image-guided techniques. The increased emphasis on prevention of these conditions also inspires me to continue my research. However, we are still challenged with what to do once the degeneration cascade begins in young, healthy individuals who are at greater risk for long-term implications if they undergo surgery. Being able to treat this subset of patients and help them avoid surgery is what motivates me the most.
The commitment of the leadership at Och Spine at NewYork-Presbyterian and Weill Cornell Medicine to fostering a culture of innovation has been crucial to our ability to engage in these innovative research projects.




