In the research labs and clinics of NewYork-Presbyterian Hospital’s extraordinary programs in inflammatory bowel disease, physicians and scientists are pursuing groundbreaking research to better understand the pathophysiology of inflammatory bowel disease that will inform the development of new treatments for the more than one million Americans suffering with ulcerative colitis and Crohn’s disease. At the same time, patients are benefitting from novel interventions and surgical approaches, as well as access to the latest clinical trials offered through these multidisciplinary centers.
A Leader in Emerging Therapeutics
Clinicians at the Jill Roberts Center for Inflammatory Bowel Disease working in synergy with scientists at the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine provide a comprehensive framework for their complementary missions of offering unparalleled patient care and pursuing clinical and scientific advances to improve outcomes across the many aspects of these diseases. Following is a review of just a few of the current endeavors with landmark potential underway by their faculty.
Ulcerative Colitis and the Role of Candida
Prior research from the Jill Roberts Center has shown that fecal microbiota transplant (FMT) can promote healing in the mucosal lining of the lower digestive tract, relieving ulcerative colitis symptoms in some people. A recent study by Weill Cornell investigators in the Jill Roberts Institute for Research in IBD presents evidence as to why higher levels of Candida in the gut are associated with better outcomes for patients with ulcerative colitis treated with FMT.
To better understand how fungi might play a role, the Weill Cornell researchers evaluated stool samples in a large, multicenter, randomized trial that analyzed 129 fecal samples from healthy donors and from ulcerative colitis patients prior to FMT or placebo treatment and then eight weeks after the procedures. The researchers also obtained serum samples from patients to study their antibody response to microbes.
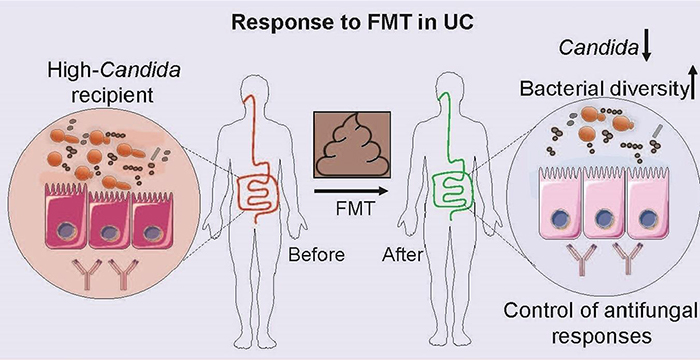
Illustration of association of Candida levels with response to fecal microbiota transplant in ulcerative colitis patients
Based on this research, published in the April 15, 2020, issue of Cell Host & Microbe, they found that patients who did not respond to the transplant had increased serum levels of antibodies to Candida. This indicated that the body was unsuccessfully trying to mount an immune response to the fungus, which in these patients might prove detrimental as an elevated immune response is part of what causes inflammation in ulcerative colitis.
The patients who benefited from this therapy had higher levels of the fungus Candida in their gut prior to the procedure. The fecal transplant acts to decrease the population of Candida, and the decline in Candida after FMT may help to reduce inflammation in the colon and rectum. Altered fungus levels may also affect bacteria in the gut, altogether reducing inflammation.
The researchers are now undertaking further studies in the clinic and the laboratory to determine what underlying biological processes explain these study findings along with better understanding of the genetic and immune makeup of ulcerative colitis patients who might respond to fecal transplant. They currently have an active clinical trial ongoing to examine the efficacy of FMT and dietary fiber in patients with ulcerative colitis (https://clinicaltrials.gov/ct2/show/NCT03998488). Their hope is that upon validation in multiple patient cohorts, such studies might one day help doctors determine which patients are candidates for FMT or other microbiome-based therapies, offering improved precision medicine approaches in the years to come.
From Trials to Treatment
More than a decade ago, faculty in the Jill Roberts Center helped to define the efficacy of interleukin-12/23 monoclonal antibody ustekinumab (STELARA®) for the treatment of moderate to severe Crohn’s disease. Most recently, in work led by Ellen J. Scherl, MD, they participated in assessing ustekinumab for ulcerative colitis through two years of maintenance therapy, demonstrating that its efficacy was sustained through 92 weeks.
The Jill Roberts Center also recently served as a lead site for the development of the sphingosine 1-phosphate (S1P) modulator ozanimod supervised by Randy Longman, MD, PhD, Director of the Jill Roberts Center. A pivotal phase 3 trial evaluating oral ozanimod (Zeposia®) as a therapy for adult patients with moderate to severe ulcerative colitis demonstrated highly statistically significant results, including induction of clinical remission at week 10 and in maintenance at week 52. The study suggests that ozanimod may address the need for new oral therapy options for patients who do not respond to other treatments. While the mechanism of action underlying the effect of ozanimod in ulcerative colitis is unknown, it may involve the reduction of lymphocyte migration into the inflamed intestinal mucosa.
Two years ago, Weill Cornell researchers in Dr. Longman's lab reported on their findings in mouse models with selective deletion of TL1A and its receptor DR3 to evaluate the role of this critical IBD-linked genetic pathway in regulating mucosal immunity. Their studies revealed a central role for IBD-linked gene TNFSF15 and its protein TL1A as a central regulator of ILC3-dependent mucosal immunity and supported a key role for IBD-associated adherent bacteria in regulating mononuclear phagocytes as the main producers of intestinal TL1A.
Building on this research and supported by grants from the NIH, the researchers continue their investigations, including participating in a phase 2a trial of PF-06480605, a first-in-class fully human immunoglobulin G1 monoclonal antibody targeting TL1A. The multicenter TUSCANY study evaluated the safety, tolerability, and efficacy of PF-06480605 in the treatment of moderate to severe ulcerative colitis. Of the 42 participants who completed the study, treatment-emergent serious adverse events were reported in 3 participants and considered treatment-related in 1 participant. At week 14, statistically significant endoscopic improvement was observed in 38.2 percent of participants, and proportions of participants achieving remission and endoscopic remission were 24 percent and 10 percent, respectively.
Advancing Novel Procedures for IBD
A new Interventional Inflammatory Bowel Disease Center and Center for Ileal Pouch Disorders at NewYork-Presbyterian/Columbia University Irving Medical Center, led by Bo Shen, MD, an internationally recognized expert in this subspecialty, is pursuing medical therapies and novel endoscopic procedures to manage complications of IBD and IBD-related surgery.
Pouchitis: Common and Complicated
Pouchitis, the most common complication among patients with ulcerative colitis who have undergone restorative proctocolectomy with ileal pouch-anal anastomosis, is actually a part of the wide spectrum of some 30 to 40 disorders that vary in etiology, pathogenesis, phenotype, and clinical course. Although initial acute episodes typically respond to antibiotic therapy, patients can become dependent on antibiotics or develop refractory disease.
A preeminent expert in pouch disorders and pouchitis, Dr. Shen has extensive experience with the pouch procedure, one of the most difficult procedures in GI surgery, which has been shown to improve patients’ quality of life significantly and to reduce the risk for colitis-associated neoplasia. At the same time, pouch surgery can result in a number of complications, including procedure-associated leaks, strictures, sinuses, fistulae, pouchitis, cuffitis, and de novo Crohn’s disease-like conditions of the pouch, and irritable pouch syndrome. The management of chronic antibiotic-refractory pouchitis has been challenging and, in fact, chronic pouchitis is one of the most common causes for pouch failure, defined as permanent diversion, pouch excision, or complete pouch revision.
Before going to reconstruction for mechanical complications such as pouch strictures, surgical leaks, and pouch prolapse, Columbia gastroenterologists have been performing the endoscopic therapy first, if possible, saving surgical intervention or revision surgery as a last step.
Columbia gastroenterologists also explored the efficacy and safety of hyperbaric oxygen therapy for chronic antibiotic-refractory pouchitis and other inflammatory conditions of the pouch. In a study published in the September 2020 issue of Inflammatory Bowel Diseases, the researchers found in a retrospective case series of adults with IBD who underwent ileal pouch-anal anastomosis and then developed chronic antibiotic-refractory pouchitis that hyperbaric oxygen therapy was well tolerated and significantly improved symptoms and endoscopic parameters.
Consensus Guidelines for Interventional Therapies
Interventional endoscopy is emerging as a treatment for IBD, particularly endoscopic procedures for Crohn’s disease-associated strictures. Most recently, Columbia faculty led the development of a consensus guideline for the endoscopic treatment of Crohn’s disease surgery published in the May 2020 issue of Lancet Gastroenterology and Hepatology. On the basis of an extensive literature review and the clinical experience of the Global Interventional Inflammatory Bowel Disease Group, Dr. Shen and his colleagues from around the world proposed detailed guidance on all aspects of the principles and techniques for endoscopic procedures in the treatment of IBD-associated strictures.
The consensus statements for endoscopic therapy of patients with Crohn’s disease-associated strictures include pre-procedural preparation, endoscopic balloon dilation and other endoscopic treatment methods, post-procedure considerations, outcomes measures, and procedure-associated adverse events and their management.
The group agrees that the goals of endoscopic treatment are to relieve obstruction and obstruction-associated symptoms, delay or prevent surgery, and improve the quality of life of patients with IBD. They recommend that the successful management of complex IBD, including Crohn’s disease-associated strictures, requires a multidisciplinary approach that draws on the expertise of IBD specialists, interventionalists, colorectal surgeons, nutritionists, gastrointestinal radiologists, and gastrointestinal pathologists.
The absence of data from large randomized controlled trials prevented the group from making strong grade A recommendations, however, the available data and collective experience of the group’s participants provide a foundation for beginning to standardize the treatment of strictures in patients with Crohn’s disease.



