For Marco B. Zoccali, MD, FACS, a colorectal surgeon at NewYork-Presbyterian/Columbia, sacral nerve stimulation (SNS) has been a routine part of the care he provides to his patients with fecal incontinence. Now, as part of a clinical trial, Dr. Zoccali is investigating the use of SNS in his patients with inflammatory bowel disease (IBD).
Dr. Marco Zoccali
The IBD Center at New York-Presbyterian/Columbia is the first academic center to offer this treatment option to patients in setting of the BOOM-IBD trial, an early feasibility study of SNS for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). In addition to Columbia, where Dr. Zoccali serves as the study’s principal investigator, there are several other leading IBD referral centers participating in the study across the country; however, Dr. Zoccali is the first to enroll patients, here in New York. BOOM-IBD is one of many trials that the IBD Center at Columbia is engaged in to improve the quality of life of millions of people living with IBD. Dr. Zoccali works closely with Bo Shen, MD, director of the IBD Center at Columbia, Ioannis Economou, MD, and Le-Chu Su, MD, PhD, to handle some of the most complex patients with IBD from across the world, thus making Columbia the perfect place to conduct this innovative study.
SNS is a type of electrical stimulation therapy in which a small device is implanted under the skin at the top of the buttocks and stimulates the sacral nerve by delivering electrical pulses. In fecal and urinary incontinence, SNS therapy can help restore normal control by modulating the nerve signaling to the pelvic organs. “The data [for SNS in incontinence] is excellent,” says Dr. Zoccali. “About 50% of patients treated will have complete [bowel] control and are able to resume a normal life. Seventy-five percent will have a remarkable improvement, meaning their quality of life gets significantly better.”
After seeing the success of SNS in fecal incontinence, Dr. Zoccali wanted to pursue alternative uses of the technology. “There is gynecological and urological literature that shows that, besides treating incontinence, you also address other symptoms, like chronic pelvic pain, sensation of incomplete evacuation, and urgency,” he says. With that in mind, he began to think about the potential application in IBD.
Urgency and stool frequency can be common in IBD; therefore Dr. Zoccali thought there could potentially be a more general application of the device to address the systemic inflammation that occurs in patients with immune-mediated conditions. He came across a study from Johns Hopkins University where researchers used SNS on rats with trinitrobenzenesulfonic acid (TNBS)-induced colitis. The researchers treated the rats with SNS, and they found that, in all the rats treated this way, their inflammation would regress. “Within 10 days, they observed that the intestinal inflammation would resolve,” says Dr. Zoccali. “However, the ones not treated [with SNS] actually got sicker and sicker from the disease that was induced.”
The study found that the anti-inflammatory effects of SNS are likely a result of the communication of the sacral nerve to the spinal cord and central nervous system back out via the vagus nerve to the target organ, or the colon in this case. It is a loop, or circuit of nerve stimulation that results in an anti-inflammatory effect. This could have potentially groundbreaking implications in the treatment of IBD in humans.
A Potential New Treatment for IBD
BOOM-IBD is an interventional, multisite clinical trial evaluating SNS to treat Crohn’s disease and ulcerative colitis using a small implantable medical device. The device, provided by Boomerang Medical, received Breakthrough Device Designation by the FDA for the treatment of Crohn’s disease and ulcerative colitis and uses gentle electrical impulses to activate nerve pathways to reduce inflammation and improve IBD symptoms.
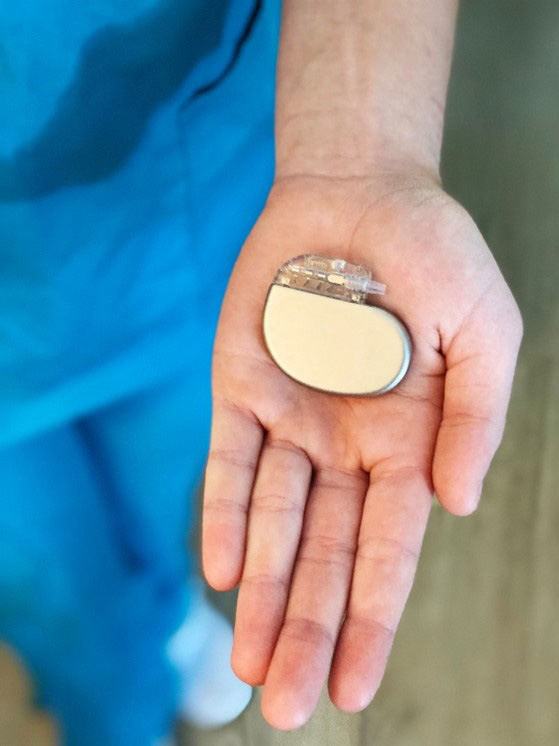
Example of the SNS device used in BOOM-IBD (Photo courtesy of Boomerang Medical).
The study is open to patients with both mild and moderate-to-severe Crohn’s disease or ulcerative colitis – as seen on endoscopy – between the ages of 18-75. “We are enrolling IBD patients that have either been exposed to medication and are not getting the appropriate response or patients that are naïve to medications and would like to explore an alternative to a pharmacological treatment” says Dr. Zoccali.
Patients who have had a previous bowel resection are eligible to participate; however, patients with an ostomy or an ileal pouch-anal anastomosis are currently excluded from the trial.
“At baseline, we are evaluating the endoscopic grading of the disease,” says Dr. Zoccali. “We assess the patient’s symptoms – urgency, the number of bowel movements, and blood in the stool. We also assess markers of inflammation in the stool and several of the inflammatory and anti-inflammatory cytokines in the bloodstream.”
After baseline evaluation, participants undergo a brief outpatient procedure under sedation during which the SNS device is implanted. “Under x-ray guidance, we thread the implant wire into the space near the sacral nerve root,” says Dr. Zoccali. “The wire attaches to a small device, implanted under the skin in the lower back where the buttock meets the iliac crest.”
The device is turned on during the procedure and participants return home the same day. They are allowed to shower the next day, but not fully submerge in water for two weeks, and can resume regular activities immediately while avoiding more strenuous exercise for the first couple weeks.
Participants’ IBD disease activity is monitored at several timepoints for the first twelve months after receiving the device. “At one month, we assess the amount of inflammation [in the intestine] via the blood and stool,” says Dr. Zoccali. “At three months, an endoscopy is repeated. And at six and twelve months, blood markers, stool markers, and endoscopy are repeated to assess ongoing disease severity.”
The objective of the study is to assess the safety and feasibility of SNS in patients with IBD. In addition, disease severity will be assessed clinically using the CDAI (Crohn’s disease activity index) score or Mayo Score (ulcerative colitis), endoscopically. In addition, histopathology will be obtained on biopsy and markers of inflammation will be obtained from blood and stool samples including fecal calprotectin, complete metabolic panel, C-reactive protein, and cytokines in plasma (Interleukin-1 beta; Interleukin-2; Interleukin-6; Interleukin-8; Interleukin-10; Interleukin-12p70; Interleukin-17A; Interleukin-21; Interleukin-23; Interferon gamma; Granulocyte-macrophage colony stimulating factor; monocyte chemoattractant protein-1; macrophage inflammatory proteins-1alpha; Transforming growth factor beta 1; Tumor necrosis factor alpha).
The first patient enrolled by Dr. Zoccali, who was undergoing treatment by Dr. Shen, has moderate disease and, despite trying multiple medications, still has inflammation present. “We don’t know if the patient is going to come off medication completely,” Dr. Zoccali says. “[I hope] this will give [them] something extra to achieve complete remission, thereby avoiding the ongoing diminished quality of life, ongoing risk of disease flares, and risk of requiring invasive surgical intervention.”
Dr. Zoccali is optimistic about the trial and its implications for IBD patients. “At this phase, we are learning which IBD phenotypes will demonstrate a consistent signal, and this information will be used to design the pivotal study,” he says. “We hope it will play an integral role in the treatment paradigm for IBD. For some, it might replace medicine. For others, it might be synergistic with IBD medication. This is what we are learning in this groundbreaking study.”
BOOM-IBD is in the early stages of recruiting participants. It is estimated that the study will be completed in 2026.





