Historically, patients with isolated tricuspid valve regurgitation have not been referred for surgical intervention but rather managed medically. Today, tricuspid insufficiency is the focus of numerous clinical trials investigating transcatheter devices and approaches for its treatment with cautious optimism. The expectation is that minimally invasive procedures for this valve will follow in the successful footsteps of transcatheter replacement of the aortic valve that has made TAVR a household name.
At NewYork-Presbyterian, Columbia, and Weill Cornell Medicine, cardiologists, interventionalists, cardiac surgeons, and cardiac imaging specialists – pioneers in the TAVR journey – are once again taking the lead through robust structural heart disease programs to pursue the next chapter in transcatheter therapies centered on the extremely challenging field of tricuspid valve intervention. An estimated 2.4 million people in the United States have significant tricuspid regurgitation (TR). However, medical therapy options are limited and despite that only about 10,000 of those undergo surgical intervention.
Dr. Susheel Kodali
Dr. Mark Reisman
Dr. Rebecca Hahn
“NewYork-Presbyterian has been involved in the evolution of structural heart disease since its inception in the early 2000s,” says Susheel K. Kodali, MD, Director of the Columbia Structural Heart and Valve Center at NewYork-Presbyterian/Columbia University Irving Medical Center. “As a result, our faculty have considerable experience in the field and continue in a purposeful and important way in its development.”
“I would describe advancements in the structural heart space as dynamic, with continual changes and refinement in technology that even for a cardiologist who lives in this space, it is frankly almost impossible to keep up,” says Mark Reisman, MD, Director of Structural Heart Disease in the Division of Cardiology at NewYork-Presbyterian/Weill Cornell Medical Center. “When a physician refers a patient to one of NewYork-Presbyterian’s structural heart team members they are going to receive a thoughtful and comprehensive evaluation of the patient and determination of the best approach to their treatment – whether it is medical therapy, catheter-based, or surgery.”
Tricuspid Valve Disease: Not to Treat is No Longer an Option
Dr. Crockatt and the other authors aimed to compare the skill acquisition and cost-effectiveness of these two forms of surgical training. Fourteen junior orthopedic surgery residents at NewYork-Presbyterian/
“Historically, it was believed that patients with tricuspid regurgitation could go without treatment or be treated medically. We generally do not operate on these patients because it’s a very high-risk surgery,” says Dr. Kodali. “However, we have realized, especially as the population is aging, that people are becoming increasingly symptomatic from tricuspid regurgitation. You try and manage their heart failure with diuretics and other drugs, but an effective medical therapy does not exist. And surgical outcomes for many patients with tricuspid regurgitation are poor with high mortality rates, which is partly why they don’t get treated. So while it is now known that there is a population with TR that does need treatment, determining who needs to be treated and what is the best way to treat these patients remains unclear.”
Dr. Reisman agrees. “Treatment for triscuspid valve disease is an area that has not been well developed, even from the surgical side, for a variety of reasons. The tricuspid valve, colloquially referred to for the longest time as the forgotten valve, poses unique challenges to interventionalists and surgeons alike. The signs, symptoms, and clinical presentation of tricuspid disease is a much more insidious process and can be very debilitating for patients. From a surgical perspective, open repair or replacement of the tricuspid valve has been fraught with complications that are very difficult to treat, such as right-sided heart failure. As a result, for the longest time, medical therapy was the standard of treatment for tricuspid symptoms.”
“Transitioning from open heart surgery to transcatheter-based approaches to treat aortic stenosis enabled patients to receive care that meets the threshold of what surgeons could achieve but with a much more minimally invasive approach,” says Dr. Reisman. “With that success and the resulting collaborations between surgical colleagues and interventional cardiologists, we have been able to expand the application of minimally invasive therapies for the mitral valve and, more recently, for the tricuspid valve.”
With cardiologists seeing more and more patients with moderate to severe tricuspid regurgitation, interest in the tricuspid valve has grown tremendously and much of that attention is on applying transcatheter therapy. In New York, Rebecca T. Hahn, MD, Director of Interventional Echocardiography for the Columbia Structural Heart and Valve Center, was involved in the study of the first transcatheter device for the tricuspid valve. While that device no longer exists, NewYork-Presbyterian’s interventional cardiologists have been leading early feasibility studies of devices for TR for several years, once again, serving as pioneers and innovators in advancing transcatheter devices and procedures for transcatheter valve therapy.
“Physicians within our group have been the national principal investigators for several different studies of tricuspid devices,” says Dr. Kodali. “Many of these devices have come and gone, but there are a number of transcatheter valve therapy systems that have shown some reasonable promise in the initial studies and are now in pivotal trials. Part of the challenge with all of these devices and therapies has been imaging, and that is an area in which we excel, including having cardiac imaging specialists on our team dedicated to transcatheter interventions.”
“The ongoing development and incredible progress in treating structural heart disease is based, in part, on the availability of advanced imaging tools for detecting structural and valve pathology, quantifying severity, and guiding therapy,” says Dr. Hahn, who was one of the earliest imagers to be dedicated to an interventional cardiology team – the brainchild of Martin B. Leon, MD, Director of Interventional Cardiovascular Care at Columbia. “Incorporating the imager into structural heart disease interventions opened the door to the potential of more complex procedures and application of new devices. The interventional echocardiographer supports pre-procedural patient selection, participates in decision-making intra-procedurally, particularly with positioning the device, and provides continuous procedural monitoring. For all these reasons the imager has become such an integral member of the heart team for structural heart disease.”
Prevailing Trials for the Tricuspid Valve
The structural heart disease teams at NewYork-Presbyterian/Columbia and NewYork-Presbyterian/Weill Cornell are united in their pursuit of transcatheter approaches and devices that can propel nonsurgical treatment of tricuspid valve disease to the next level.
“NewYork-Presbyterian has been leading the way with several protocols for treatment of the tricuspid valve,” says Dr. Reisman. “Transcatheter therapies now have progressed to a point that we have been able to begin to offer patients mechanical repair or replacement of their tricuspid valve.”
“At NewYork-Presbyterian, we have been at the forefront of transcatheter tricuspid valve therapy, and Columbia was one of the first sites in the country to perform a transcatheter tricuspid valve intervention,” says Dr. Kodali. “If there is a patient who has been told that there is nothing that can be offered for their tricuspid disease or who has been turned away from institutions that do not offer these types of trials, it is worthwhile having them evaluated at NewYork-Presbyterian, where several clinical trials are underway.”
Columbia and Weill Cornell Medicine faculty are leading investigators in the following pivotal trials – one for tricuspid valve repair and a second trial for tricuspid valve replacement.
Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical Trial (CLASP II TR)
Patients with severe, symptomatic tricuspid regurgitation that persists despite medical therapy may be candidates for the CLASP II TR clinical trial to determine the safety and effectiveness of the PASCAL Transcatheter Valve Repair System. Dr. Martin Leon serves as one of the two national Principal Investigators for the trial, along with Michael J. Mack, MD, at Baylor Scott & White Health. Dr. Susheel Kodali serves as Principal Investigator for the Columbia trial site and Shing-Chiu Wong, MD, Director of Cardiac Catheterization Laboratories at NewYork-Presbyterian/Weill Cornell, serves as Principal Investigator for the Weill Cornell Medicine trial site.
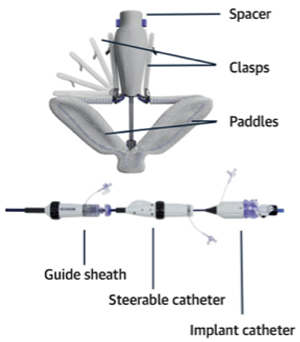
PASCAL is a leaflet repair system to treat TR and is implanted under transesophageal echocardiography and fluoroscopic guidance. The implant consists of a central spacer and adjacent paddles and clasps that attach the implant to the native leaflets to reduce regurgitation. The goal is to implant the device at the site with the largest regurgitant lesion and reduce leak.
The PASCAL implant contains two broad paddles with clasps that can be simultaneously or independently maneuvered for tricuspid valve leaflet capture. (Credit: Edwards Lifesciences)
The prospective pivotal trial takes place at 48 sites, with a recruitment target of 825 patients. Started in December 2019 with a primary completion date in December 2024, the study is evaluating the safety and effectiveness of transcatheter tricuspid valve repair with the PASCAL system and optimal medical therapy compared to optimal medical therapy alone. TR severity is assessed by either transthoracic echocardiography or transesophageal echocardiography. Patients will be seen for follow-up visits at discharge, 30 days, 3 months, 6 months, and annually through 5 years. Primary outcome measures include all-cause mortality, RVAD implantation or heart transplant, tricuspid valve intervention, heart failure hospitalizations, and quality-of-life improvements at 24 months, with a secondary measure focused on reduction in TR severity at 12 months.
In the February 2, 2021, issue of the Journal of the American College of Cardiology, Dr. Kodali, who was lead author on the paper, Dr. Hahn, Dr. Leon, and their colleagues at seven U.S. investigational sites across the country reported on the first 30-day outcomes of the early feasibility study of PASCAL, which had previously received approval for use in Europe. A total of 34 patients with a mean age of 76 years were enrolled in the study. Findings at 30 days for the 29 patients (85 percent) who received implants included:
- 85 percent of patients achieved a TR severity reduction of at least 1 grade, with 52 percent achieving moderate or less TR
- Major adverse event rate was 5.9 percent; none of the patients experienced cardiovascular mortality, stroke, myocardial infarction, renal complication, or reintervention
- 89 percent of the patients improved to NYHA functional class I/II
- Mean 6-minute walk distance improved by 71 meters
- Mean Kansas City Cardiomyopathy Questionnaire (KCCQ) score improved by 15 points
The investigators concluded the PASCAL transcatheter valve repair system performed as intended, with substantial TR reductions and favorable safety results, and paved the way for CLASP II TR, now underway.
TRISCEND II Pivotal Trial: EVOQUE System for Transcatheter Tricuspid Valve Replacement
The TRISCEND II study is evaluating the safety and performance of transfemoral transcatheter tricuspid valve replacement in patients with clinically significant tricuspid regurgitation and elevated surgical risk. Dr. Kodali and Dr. Hahn are two of the four Principal Investigators for the 51-site trial in the United States and Germany. Dr. Reisman serves as the Principal Investigator for the Weill Cornell Medicine investigational site.
The TRISCEND II clinical trial is based on the results at 30 days of the early feasibility study led by Dr. Kodali and conducted at 14 sites with an enrollment of 132 patients. Those findings, published in the March 14, 2022, issue of JACC. Cardiovascular Interventions, demonstrated technical feasibility and safety, as well as reduction of TR and improvement in symptoms. “We saw high rates of device and procedural success – 98.2 percent and 96.4 percent, respectively, with low mortality and significant TR reduction,” says Dr. Kodali. “Even at 30 days, what's remarkable is that all patients had at least a one grade reduction, 95 percent had a two grade reduction, and more than a quarter had a four grade reduction in tricuspid regurgitation. And these are patients that presented with massive and torrential TR and they still tolerated the procedure well. Importantly, these acute outcomes were associated with significant improvements in functional status and quality of life.”
The unique design of the EVOQUE implant engages leaflets, chords, and annulus to achieve secure placement. (Credit: Edwards Lifesciences)
At six-month follow-up in 56 patients, Dr. Kodali and his team found these results were sustainable, including:
- Transfemoral tricuspid valve replacement with the EVOQUE system demonstrated favorable 30-day outcomes that were sustained at 6 months
- Significant TR reduction by core laboratory assessment, with 100 percent of patients having mild or less TR
- 96 percent survived and 94 percent had freedom from heart failure hospitalization
- Significant improvements in NYHA class, KCCQ score, and 6-minute walk distance
A second study, also published in the same issue of JACC. Cardiovascular Interventions, reported on one-year results from an expanded first-in-human experience with the EVOQUE system, in which 27 patients with severe TR were treated in a compassionate-use experience at seven centers. All of the patients had clinical right-sided heart failure and were deemed inoperable and unsuitable for transcatheter edge-to-edge repair by the institutional heart teams. The study showed:
- One-year mortality of 7 percent (2/27)
- 70 percent of patients were NYHA functional class I/II
- 96 percent and 87 percent of patients had a TR grade ≤2+ and ≤1+, respectively, at 1 year
- Between 30 days and 1 year, 2 patients experienced heart failure hospitalizations, and 1 patient required a new pacemaker implantation
Based on the results of each of these early studies, the TRISCEND II clinical trial is also well underway.