Genetics research has illuminated the origins of hypertrophic cardiomyopathy (HCM), the most common inherited cardiovascular disease in the United States. However, the molecular signaling pathways that lead to the diverse clinical manifestations of HCM, including arrhythmia, stroke, heart failure, and sudden cardiac death, have remained obscure.
Elucidating the signaling pathways from gene mutation to clinical expression is the research aim of Yuichi J. Shimada, MD, MPH, a cardiologist and research director of the Hypertrophic Cardiomyopathy Center at NewYork-Presbyterian and Columbia. In the most comprehensive, large-scale study to date, Dr. Shimada and his colleagues found newly recognized signaling pathways, such as Ras-mitogen-activated protein kinase (Ras-MAPK), that are differentially regulated in HCM.
Below, Dr. Shimada discusses the study findings, which offer hope for the development of therapies that promise to halt, or even prevent, this complex disease.
Current therapeutics for HCM are not as effective as we would wish because we haven't cracked the black box – the molecular target – that we need therapies to reach. Moreover, the signaling pathways that control the progression of HCM from mild to severe disease are largely unknown.
— Dr. Yuichi Shimada
From Gene Mutation to Clinical Expression
Scientists have already found more than 15 genes and more than 1,800 gene mutations responsible for HCM, and the clinical phenotype of HCM has been extensively studied. However, few studies have identified the mechanical link between these gene mutations and the clinical expression of the disease. As a result, current therapeutics for HCM are not as effective as we would wish because we haven't cracked the black box – the molecular target – that we need therapies to reach. Moreover, the signaling pathways that control the progression of HCM from mild to severe disease are largely unknown.
Research Goals
Through this research, we sought to gain a better understanding of the pathogenesis and risk factors for the progression of HCM at the molecular level to inform the development of new therapies for the disease. In previous studies, our group had analyzed blood plasma to identify the molecular pathways associated with HCM disease progression. However, blood samples can only provide indirect information about what’s going on in a particular organ, so it’s a huge assumption to say we can tell what’s going on in the heart by analyzing blood plasma. For this study, we analyzed samples of myocardial tissue, which can provide us with direct evidence of what is happening in the heart.
Our first research aim was to compare the myocardial tissue of people with HCM disease versus normal healthy individuals to identify the signaling pathways that are differentially regulated in HCM. The second aim was to associate these signaling pathways with the clinical indicators of severe disease to explain why some patients get severe HCM and some patients get less severe HCM, because that's another mystery we hoped to unravel.
Research Methods
In this multicenter, case-controlled study of cases with HCM (N=99) and controls with non-failing hearts (N=15), we performed proteomic profiling of more than 7,000 proteins from myocardial tissue samples in the largest study of patients with HCM to date. Next, we performed a pathway analysis of differentially expressed proteins (DEPs) whose concentrations correlated with five different clinical parameters of severe HCM (such as reduced left ventricular ejection fraction, atrial fibrillation, and ventricular tachyarrhythmias). Finally, we confirmed the findings from the proteomic profiling using myocardial transcriptomic profiling, the quantification of more than 30,000 gene expression in tissue samples at the transcription (RNA) level, as transcriptomics provides additional layer of biological information.
Our analysis identified that Ras-MAPK is activated in the heart of patients with HCM. Ras-MAPK is upregulated in the myocardium of HCM cases compared to controls, especially in more severe cases of HCM. We demonstrated the upregulation of the same newly recognized pathway in association with the clinical indicators of severe HCM, and further validated these findings through myocardial transcriptomic profiling.
— Dr. Yuichi Shimada
Key Findings
Our analysis identified that Ras-MAPK is activated in the heart of patients with HCM. Ras-MAPK is upregulated in the myocardium of HCM cases compared to controls, especially in more severe cases of HCM. We demonstrated the upregulation of the same newly recognized pathway in association with the clinical indicators of severe HCM, and further validated these findings through myocardial transcriptomic profiling.
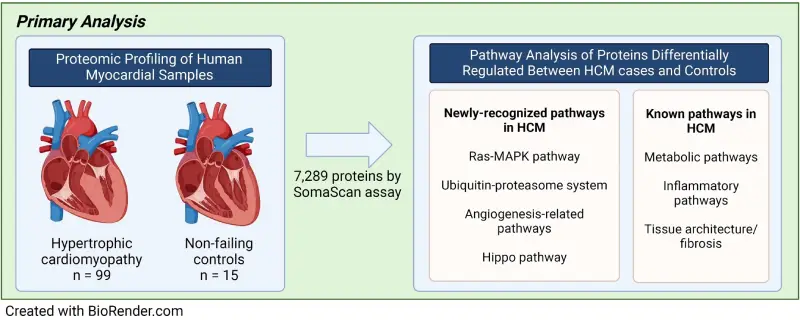
Researchers identified dysregulation of both newly recognized and known pathways associated with HCM pathogenesis and progression. These pathways could inform future efforts to develop targeted therapeutics for HCM. Image from BioRender.
Identifying the Molecular Target of HCM
What is so exciting about these findings is we have identified the molecular target of HCM, the Ras-MAPK pathway, which has promising implications for development of precision medications to treat this complex disease.
We know that in diseases other than HCM, the activation of the Ras-MAPK pathway also causes abnormal heart thickening of the heart muscle. For instance, Ras-MAPK activation causes Noonan Syndrome, a genetic disorder that affects the heart, blood vessels, eyes, ears and brain. There are medications available to inhibit the Ras-MAPK pathway, which may have therapeutic implications for HCM.
This gives us a golden opportunity to intervene with new therapies for HCM to prevent the manifestation of the disease. Even for someone who has already developed HCM, these therapies can prevent the disease progression to more severe cardiovascular events, such as sudden cardiac death.
— Dr. Yuichi Shimada
A Golden Window for Intervention
As clinicians, we recommend genetic testing for all family members of an individual with HCM because each first-degree family member has a 50% chance of inheriting the pathologic gene mutation. In this country and worldwide, there are many patients who are deemed to carry a pathogenic gene mutation but have not yet developed the disease. This gives us a golden opportunity to intervene with new therapies for HCM to prevent the manifestation of the disease. Even for someone who has already developed HCM, these therapies can prevent the disease progression to more severe cardiovascular events, such as sudden cardiac death.
On a Path to Precision Medicine for HCM
Through NewYork-Presbyterian and Columbia’s Hypertrophic Cardiomyopathy Center, we bring together all of the experts in one setting to provide patients with the most comprehensive care, including participation in research studies and clinical trials like this one.
Now that we have identified the molecular target for HCM, the next natural step would be to conduct a study to test whether inhibiting Ras-MAPK can reverse or prevent the development and progression of the disease.
HCM is known to be a very heterogeneous disease, so some patients will have Ras-MAPK activation, and some patients will not. So, putting this information into practice, we would identify HCM patients who have activation of the Ras-MAPK pathway, and then treat these patients with the Ras-MAPK inhibitor. That's truly precision medicine.




