In a field of rapidly developing techniques and technologies, left ventricular assist devices (LVAD) have revolutionized treatment options for patients with heart failure. The newest generation LVAD, the HeartMate 3 (HM3), extends length and quality of life in end-stage heart failure patients. However, there have been reports of outflow graft narrowing in the LVAD due to the accumulation of biodebris.
To better understand the clinical significance of this problem, NewYork-Presbyterian heart failure specialists Yoshifumi Naka, MD, PhD, Surgical Director of Heart Failure, Heart Transplantation, and Mechanical Circulatory Support Programs, and Nir Uriel, MD, MSc, Director of Advanced Heart Failure and Cardiac Transplantation, collaborated with Jay S. Leb, MD, Director of Cardiac Imaging at NewYork-Presbyterian/
Outflow graft narrowing is the result of the leakage and accumulation of plasma between the outflow graft and the bend relief of the device. At this point, we don’t fully understand why this happens, and why certain patients develop the narrowing and why others don’t.
— Dr. Yoshifumi Naka
The Advancements and Risks in Innovative Technology
Originally considered lifesaving therapy for patients ineligible for heart transplantation, LVADs are now used to assist in heart recovery and as a destination therapy for end-stage heart failure patients. Recently, the pivotal Multicenter Study of MagLev™ Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3™ (MOMENTUM 3) demonstrated that the five-year survival for patients – nearly 60% – is approaching the five-year survival rates of heart transplant recipients who have a similar risk profile. The study also compared the newest LVAD to its predecessor when used for the treatment of advanced refractory left ventricular heart failure and found that the newest device was associated with less need for pump replacement, lower risk of bleeding or pump thrombosis, and fewer days in the hospital. Despite these improvements, outflow graft narrowing remains a formidable risk.
“Outflow graft narrowing is the result of the leakage and accumulation of plasma between the outflow graft and the bend relief of the device,” explains Dr. Naka. “At this point, we don’t fully understand why this happens, and why certain patients develop the narrowing and why others don’t.”
“This study, which is a unique collaboration between the cardiology and the heart imaging teams at NewYork-Presbyterian, sought to understand the prevalence and clinical outcomes of biodebris accumulation in patients with the latest LVAD,” says Dr. Uriel, a principal investigator of the MOMENTUM 3 trials. “When it became apparent to us that outflow graft narrowing is a potentially significant issue in patients, I called Dr. Leb and said we should build a relationship between the imaging and cardiology teams to get to the root of this problem.”
This study underscores the importance of a robust partnership between the cardiology and the cardiac imaging teams, which is essential to a successful heart failure program.
— Dr. Nir Uriel
The researchers conducted a retrospective study of 165 patients who received an HM3 LVAD at NewYork-Presbyterian/
Out of 165 LVAD recipients, 46 (28%) had imaging that qualified them for study inclusion. Outflow graft narrowing was present in 15 out of 46 patients (33%). The researchers noted that outflow graft narrowing was significantly associated with a longer duration of LVAD support (588.2 ± 277.5 days vs 131.5 ± 170.9 days; P < .0001). One-year survival after identification of narrowing was 93%, with death occurring in 1 patient with complete obstruction. LV unloading (mean percent decrease in LV end-diastolic diameter at time of CT imaging vs pre-LVAD) was 16.7% vs 17.7% in patients with and without narrowing, respectively (P = .86).
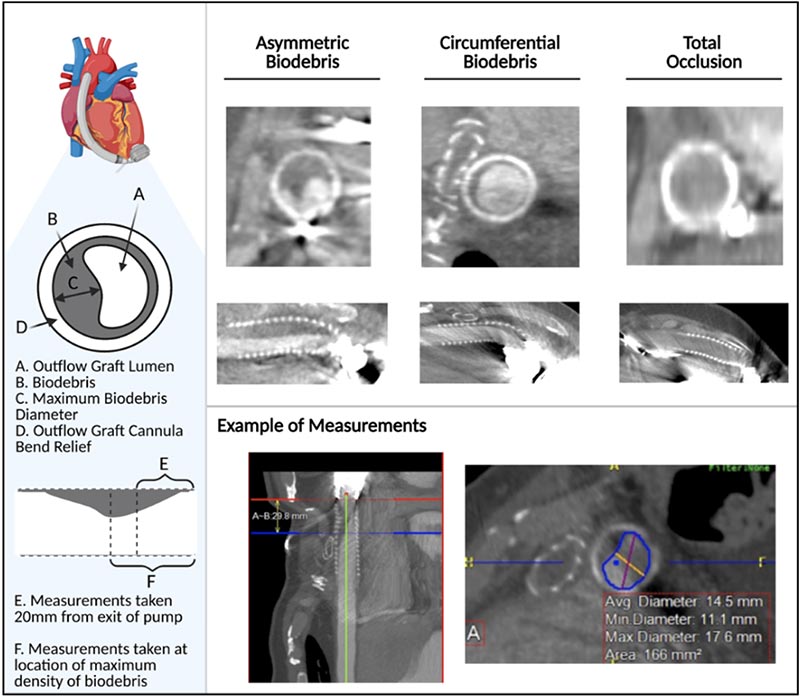
Approach to Imaging Analysis
The researchers concluded that among the LVAD recipients who underwent adequate imaging of the outflow graft, one-third had evidence of biodebris buildup. The outflow graft narrowing was associated with a longer duration of LVAD support, but it did not significantly impact LVAD unloading (output). Further studies are needed to understand the long-term impact of biodebris accumulation and to determine the need for and duration of interval CT screenings in these patients.
We know today that patients can be supported for a long time on LVADs. From this study, we learned that among 46 patients who had the CT test, 33% had narrowing, but the majority did not have clinically significant narrowing. This is because of the ability of the LVAD pump to override partial obstruction.
— Dr. Nir Uriel
Understanding the Long-Term Impacts of LVADs
“We know today that patients can be supported for a long time on LVADs,” says Dr. Uriel. “From this study, we learned that among 46 patients who had the CT test, 33% had narrowing, but the majority did not have clinically significant narrowing. This is because of the ability of the LVAD pump to override partial obstruction.”
“We also learned from this study that there is no specific age, gender, or comorbidity that is a risk factor for outflow obstruction,” continues Dr. Uriel. “This means we must go to the clinical scenario to detect a change in the pump’s performance (the power, speed, or flow). If there is a clinical concern that the heart is not unloading, or if we notice abnormal features of pump and have a low index of suspicion that there is a problem, then we should perform a CT scan to avoid an event.”
“I was actually surprised that up to one-third of patients already had biodebris buildup,” says Dr. Naka. “We hope to increase awareness among physicians that this potential problem exists in patients.”
“This study underscores the importance of a robust partnership between the cardiology and the cardiac imaging teams, which is essential to a successful heart failure program,” adds Dr. Uriel.





