As the Vice Chairman of the Department of Neurological Surgery and Director of Pediatric Neurological Surgery at NewYork-Presbyterian/Weill Cornell Medicine, Mark Souweidane, MD, has encountered some of the most insurmountable diagnoses. But that has not derailed his commitment to finding new, innovative ways to treat rare, and potentially fatal, pediatric brain tumors. One of the biggest challenges he seeks to overcome is how to break the blood brain barrier to get treatment directly to the source.

Dr. Mark Souweidane
Dr. Souweidane has pioneered clinical trials testing the use of convection-enhanced delivery of chemotherapy in diffuse intrinsic pontine gliomas (DIPG) and now, he has applied learnings from that trial to innovate treatment of choroid plexus carcinoma in partnership with Y. Pierre Gobin, MD, Director of Interventional Neuroradiology at NewYork-Presbyterian/Weill Cornell Medicine.
Below, Dr. Souweidane discusses this disease, his current work with intra-arterial chemotherapy, and the story of the first enrolled patient in his groundbreaking choroid plexus carcinoma trial.
What is choroid plexus carcinoma and what was the driving force in your pursuit of treating such a rare and devastating condition?
Choroid plexus carcinoma are extremely rare tumors that occur in early childhood, often in infancy, and develop in the ventricles of the brain. Traditionally, neurosurgeons and neuro-oncologists have attacked these tumors via surgical removal followed by adjuvant chemotherapy and/or radiation.

Example of a choroid plexus carcinoma presenting with symptoms of hydrocephalus
However, surgical removal of choroid plexus carcinoma is extremely complex as we are forced to balance the low circulating blood volume with the large voluminous hypervascular tumors. Unfortunately, up to 10% of children lose their lives just due to the surgical interface from blood loss and fifty percent leave the operating room with a subtotal resection, destined to fail adjuvant therapy. For those who do survive surgery and subsequent treatment, three-year survival hovers around 30-40%. I have always thrived on the challenge and continue to be motivated to find ways to improve these outcomes and give children a better chance at long-term, meaningful survival.
What inspired the design of your trial for intra-arterial chemotherapy for choroid plexus carcinoma?
NewYork-Presbyterian has a long history of working with intra-arterial chemotherapy and Dr. Gobin is a pioneer of this work for the treatment of ocular retinoblastoma (Rb). In Rb, tiny, hair sized catheters are threaded into the thalamic artery, delivering medication directly into the Rb and thereby treating the disease and salvaging the eye.
When I was thinking of what tumor we could apply intra-arterial chemotherapy in next, it made sense to try choroid plexus carcinoma. Choroid plexus carcinoma is like Rb in its anatomy and shares similar biological mechanisms with respect to their chemosensitivity to drugs. In this phase I trial, we are delivering concentrated doses of intra-arterial chemotherapy directly into the choroid plexus tumors to shrink them, reduce blood flow, and get them through second-look surgery safer and more effectively.
You enrolled and successfully completed treatment on your first patient — Aiden — in this trial. Can you share a little bit about Aiden and what made him a good candidate for the trial?
Aiden Rodriguez is the first patient in the world ever treated with intra-arterial chemotherapy for choroid plexus carcinoma. He is a seven-year-old who was initially diagnosed in October of 2022 after presenting with symptoms of hydrocephalus – nausea and vomiting – and then, according to his mother, he was exhibiting some very aggressive behavior, which was atypical for him. His local oncologist in upstate New York attempted to excise the tumor three times but was unable to do so due to the extreme complexity. In fact, before coming to NYPH, previous attempts for tumor removal resulted in massive needs for blood transfusion and severe life-threatening complications.

Aiden Rodriguez is the first patient in the world ever treated with intra-arterial chemotherapy for choroid plexus carcinoma.
His mother came across our trial online while looking for other treatment options. She brought it to Aiden’s local oncologist who got in touch with me, and we coordinated his treatment from there.
Aiden came to NewYork-Presbyterian in May 2023 for the trial. His participation began with an angiogram under anesthesia, which was performed by Jared Knopman, MD, Director of Cerebrovascular Surgery and Interventional Neuroradiology at NewYork-Presbyterian/Weill Cornell Medicine. During the angiogram, Dr. Knopman assessed Aiden’s angiographic pattern and found that Aiden was a very good candidate for intra-arterial chemotherapy as he had two very good blood vessels that went to the tumor and provided blood flow to it. His anterior and posterior choroidal arteries were accessible and a good enough size for us to use our smallest microcatheters to deliver the chemotherapy through the blood vessels to the tumor bed.
What was were the stages of treatment for Aiden in the trial?
We decided on the spot during the angiogram to proceed forward with the intra-arterial chemotherapy based on his vasculature. Dr. Gobin used microcatheters to infuse three medications – melphalan, carboplatin, and topotecan – through the posterior and anterior choroidal arteries directly into the tumor. Aiden went to the ICU for monitoring overnight and was released the next day.
A month later, he came back to NewYork-Presbyterian, and we did another angiogram. Initially, I had asked Dr. Knopman do an embolization to shut down the blood supply to the tumor to make surgery safer. However, when we did the new imaging, we saw that the chemotherapy had decreased the tumor blush significantly, and embolization wasn’t needed. The next day, I performed a right parietal craniotomy and successfully obtained a gross total excision of the tumor.

The intra-arterial chemotherapy protocol for the clinical trial and results from the subsequent surgery.
Getting Aiden through the surgery was incredibly exciting. There was negligible blood loss without any need for blood transfusion, and he came out looking like a superstar. He remained in the hospital for a few days of monitoring and then was released to go home and back into the care of his local oncologist.
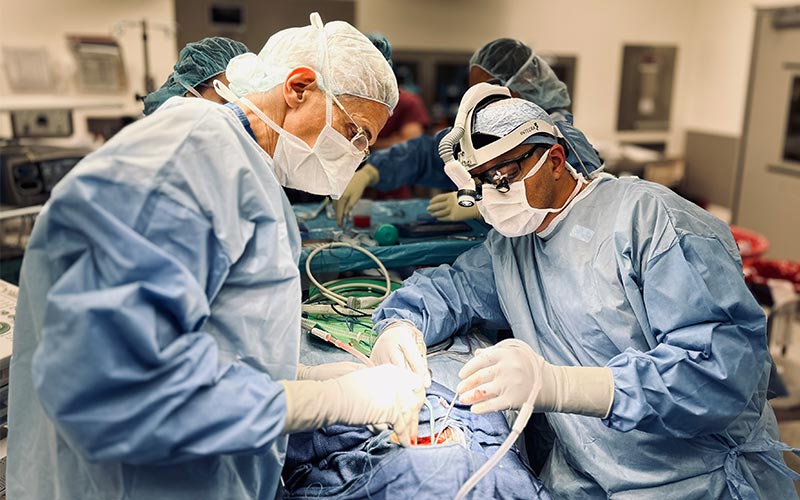
Dr. Mark Souweidane and Dr. Gary Kocharian, neurosurgery chief resident and endovascular neurosurgery fellow, performing surgery in the operating room on Aiden.
How is Aiden doing now?
Aiden is doing great. He’s been deemed no evidence of disease going into adjuvant therapy. This alone, provides him with the best prognosis for durable control and potential cure. Of course, his is family is thrilled. He’s back in school. He has a long road ahead of him but from where he was when he came here to how he is doing now, I don’t think that anyone expected he would be doing so well.

Aiden recovered and healthy after the IAC clinical trial.
What are the next steps for this trial?
Our primary objectives are to achieve reduced surgical blood loss, greater extent of tumor excision, and volumetric reduction based on MRI, all which were achieved with Aiden’s treatment. We are the only site in the world conducting this Phase I trial and we are hoping to enroll six children in it. However the infrequent nature of these tumors has really stagnated forward progress in research and clinical work. Unless someone is already aware of the trial, like an oncologist, it’s a challenge to reach the doorstep of whomever is caring for that child. We’re aggressively promoting the trial and conducting outreach. Our hope is to reach a worldwide community of people looking for a better outcome for children with this rare tumor so that we can get more patients enrolled and treated while demonstrating safety and efficacy.




