A dual-chamber leadless pacemaker system demonstrated atrial pacing and reliable atrioventricular synchrony without serious device- or procedure-related adverse events in the first three months after implantation, according to a large international study that included James E. Ip, MD, FACC, FHRS, Director of Cardiac Pacing and Implantable Devices at New York-Presbyterian/

James E. Ip, MD, FACC, FHRS
The results, among the first 300 patients enrolled in the trial, were announced at the 2023 Heart Rhythm Society annual meeting and simultaneously published in the New England Journal of Medicine.
The success of this investigational device could someday expand access to innovative leadless technology for patients who currently are implanted with conventional dual-chamber pacemakers, which represents about 80% of pacemakers in the US. Unlike leadless systems, which are fully self-contained, conventional transvenous cardiac pacemakers are significantly larger and have transvenous leads, a surgical pocket, and a pulse generator, all of which are associated with potential short and long-term complications.
“The Achilles heel of the pacemaker system is the lead itself, which can fracture with time. If you bend a wire enough times, it can break,” says Dr. Ip.
Some patients are not candidates for conventional pacemakers because they have vascular access issues that prohibit the placement of a pacemaker from the upper chest. In addition, some patients are at high risk of infections with traditional pacemaker because of prior device infections or other medical conditions.
The US Food and Drug Administration approved the first leadless pacemaker, Medtronic’s Micra Transcatheter Pacing System, in 2016, followed in 2022 by the approval of Abbott Medical’s Aveir leadless pacemaker system. Both of the devices currently on the market are single-chamber leadless pacemakers, which represent a minority of pacemakers implanted.
The Achilles heel of the pacemaker system is the lead itself, which can fracture with time. If you bend a wire enough times, it can break.
— Dr. James Ip
Development of the dual-chamber leadless pacemaker is the critical next step, making the advantages of leadless technology available to the majority of patients who require dual-chamber devices that either stimulate the atrium or synchronize the upper and lower chambers of the heart, Dr. Ip says.
Following successful testing in preclinical models, the Aveir DR i2i trial evaluated the next-generation Abbott leadless pacemaker system in humans for the first time. The study included adult patients with a conventional indication for dual-chamber pacing, most commonly sinus node dysfunction and atrioventricular block. Investigators implanted the leadless system, which consists of two separate pacemaker devices, percutaneously by a catheter manipulated through the femoral vein and into the target chambers. Both devices were inserted in a single procedure using fluoroscopy, with optional use of contrast injection and intracardiac echocardiography.
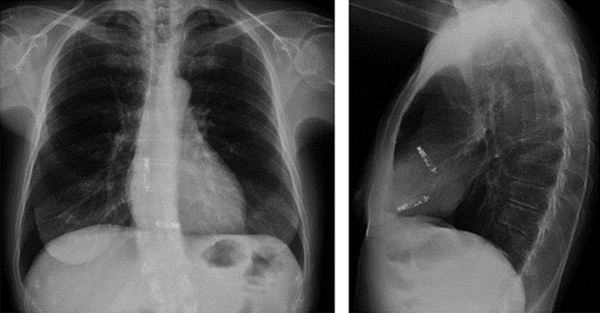
A chest X-ray showing a dual-chamber leadless pacemaker.
As part of the multicenter, international trial, Dr. Ip and his team at New York-Presbyterian/
After three months, more than 90% of patients had experienced no procedural or device complications, exceeding the preset safety goal. Other notable safety data include:
- Overall, 35 complications occurred among 29 patients
- Two patients (<1%) experienced pericardial effusion related to the atrial pacemaker
- Six intraprocedural dislodgements occurred (five atrial, one ventricular) among five patients (<2%)
- Four deaths occurred during follow-up but were not deemed to be related to the device or the procedure
“Most procedural complications will occur within the first week, so the first three months offered a good window to see if the system was safe and effective. We know from the single-chamber leadless pacemaker that if the device is working during the first three months, there’s typically no long-term failure with these devices,” Dr. Ip says.
Overall, dual-chamber leadless pacemakers are safe and effective and equivalent to a regular pacemaker.
— Dr. James Ip
In terms of effectiveness, the study’s primary performance endpoint was a combination of adequate atrial capture threshold and atrial sensing amplitude. More than 90% of patients met the endpoint, exceeding the preset performance goal, and no patients required a system revision because of inadequate atrial sensing. The dual-chamber leadless pacemaker system also met another performance endpoint, a measure of atrioventricular synchrony performance. More than 97% of patients achieved at least 70% atrioventricular synchrony, exceeding the preset goal.
“Overall, dual-chamber leadless pacemakers are safe and effective and equivalent to a regular pacemaker,” Dr. Ip says.
The trial will continue, with non-U.S. sites continuing to enroll patients. The research group will also be publishing additional data, including long-term outcomes as they follow patients for up to 12 months.
Dual-chamber leadless pacing technology has clear advantages, from increasing access for patients with vascular problems to reducing the risk of lead fractures and infections over conventional options. The leadless pacemaker also eliminates some of the appearance and discomfort issues of the larger conventional pacemaker. “It’s a very exciting time,” Dr. Ip says. “We haven’t had much innovation in pacemakers until recently.”




