|
||||||||||||||||||||||||||||||
|
Ovarian Cancer: Overcoming Obstacles to Immunotherapy
The promise of immunotherapy is altering how many cancers are perceived and treated, but it has so far eluded the treatment for some of the more aggressive malignancies, including ovarian cancer. “The same therapeutic approaches for ovarian cancer have been used for the last 40 years, but survival for patients with metastatic ovarian cancer, which is the most common stage at which it is diagnosed, has not substantially improved,” says Juan R. Cubillos-Ruiz, PhD, Assistant Professor of Microbiology and Immunology in Obstetrics and Gynecology at Weill Cornell Medicine. “Less than 27 percent of the patients diagnosed with metastatic ovarian cancer will live five years. When patients are diagnosed with localized or primary disease, their prognosis for survival is about 90 percent. The problem is that only 20 percent of the tumors are actually detected at that early stage. The others are detected when the tumor has already spread throughout the peritoneal cavity. At that stage, the cancer cells are refractory to chemotherapy. It’s a very aggressive disease and the progression is extremely accelerated.”
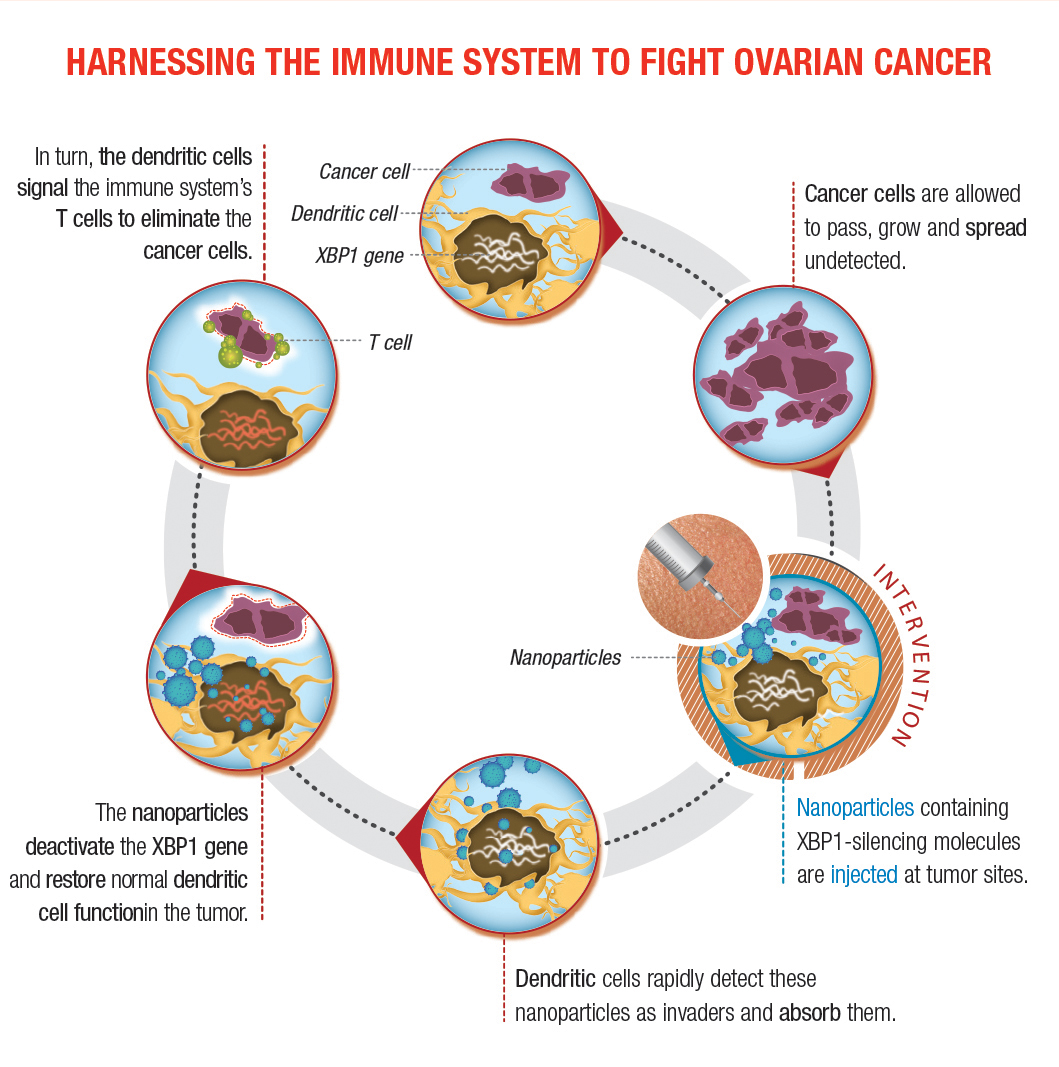
Not Your Average Immunosuppression For the past decade, Dr. Cubillos-Ruiz has focused his scientific career on understanding why the immune system is unable to control this cancer. “While immunotherapy is eliciting remarkable responses against some tumor types, the same immune-based approaches are not working well in ovarian cancer or other cancers that are more aggressive,” says Dr. Cubillos-Ruiz. “These tumors, especially in ovarian and pancreatic cancer, are extremely immuno-suppressive. They have evolved multiple mechanisms to cripple immune cell functions, inhibiting the capacity of the immune system to eliminate the cancer cells. For example, while checkpoint blockade inhibitors for metastatic melanoma induce significant responses in 30-40% of patients, the same strategy is partly effective only in 10% of metastatic ovarian cancer patients.” In light of the shortcomings of current immunotherapeutic approaches for these more aggressive tumors, Dr. Cubillos-Ruiz and his research group have been analyzing what transpires in tumors from ovarian cancer patients. “We are profiling and interrogating the function of those immune cells inside the tumor to understand why they are able to infiltrate the tumor, but not function,” he explains. “What we have found is that they enter into an adverse, very hostile microenvironment in which nutrients are lacking. There’s little glucose. There’s also hypoxia, pH stress, and oxidative stress. We found that this combination of harsh environmental factors provokes accumulation of misfolded proteins in the endoplasmic reticulum [ER] of infiltrating immune cells, thereby inducing a cellular state called ‘ER stress.’ During this process, the IRE1α-XBP1 signaling pathway of the unfolded protein response is aberrantly activated and this ultimately inhibits the development of an effective anti-tumor immune response.” Cancer cells are known to exploit the IRE1α-XBP1 arm of the ER stress response to efficiently adjust their protein-folding capacity and ensure survival under hostile tumor microenvironmental conditions, says Dr. Cubillos-Ruiz. “However, we found that dendritic cells residing in the ovarian cancer microenvironment also experience severe ER stress and demonstrate persistent activation of the IRE1α-XBP1 pathway. “The unfolded protein response should be intermittent, activating and then shutting down,” continues Dr. Cubillos-Ruiz. “It should be very specific in terms of time and strength. But the problem is that when this pathway is persistently on it causes immune cell dysfunction. We call this event ‘abnormal ER stress responses’ because the pathway is trying to correct and improve the folding of the proteins, but at the same time is inducing collateral damage in the immune cells.” Dr. Cubillos-Ruiz and his colleagues turned their investigation to mouse models of ovarian cancer. Making use of elegant genetic systems developed in the lab, they were able to specifically abrogate the IRE1α-XBP1 pathway in immune cells. “We developed ovarian cancers in mice devoid of ER stress sensors specifically in immune cells to determine what would happen with disease progression in this context,” says Dr. Cubillos-Ruiz. “The immune cells were still able to enter the tumor, but by turning off the genes encoding either IRE1α or XBP1, they did not react to the inhospitable environment. Because they lacked those stress sensors they were refractory to factors that otherwise would inhibit their function, providing them with a more potent, enhanced anti-tumoral function.” Importantly, Dr. Cubillos-Ruiz notes, he and his research team were able to confirm the mechanistic and functional findings found in the mouse model using human samples from ovarian cancer patients. “We wanted to make sure that this new process was also taking place in freshly isolated human specimens, and we demonstrated that the ER stress response pathway was indeed active in the immune cells that infiltrated human ovarian tumor cells as well.” Transitioning to Therapeutic Mechanisms Having described how the previously unrecognized process of ER stress disrupts metabolic homeostasis and antigen-presenting capacity in dendritic cells, Dr. Cubillos-Ruiz and his team now had what they needed to begin pursuing therapeutic implications. “In our first approach we are developing small molecule inhibitors for ER stress sensor,” he says. “These small molecule inhibitors will bind the sensor, IRE1α, in vivo, in the tumor, and will prevent their activation. We hope that treatment with these inhibitors would mimic what we found in our gene-deficient animals – if the immune cells can’t sense ER stress, they can function better. We are now trying to devise first-in-class drugs that can inhibit the IRE1α-XBP1 pathway in both cancer cells and dendritic cells, which would sensitize the cancer to treatment and restore an immune response against it.” Their second approach involves nanoparticles that encapsulate small interfering RNA. Dr. Cubillos-Ruiz and his research team have tested a strategy in which mice were injected with nanoparticles he developed. These microscopic polymers encapsulate nucleic acids that can silence the genes encoding IRE1α or XPB1. Dendritic cells in the tumor detect the nanoparticles as invaders and ingest them. Once inside, the nanoparticles deliver the molecule that turns IRE1α-XPB1 signaling off, allowing dendritic cells to instruct the immune system to attack the cancer. “The nano-particles act like a Trojan Horse, releasing the payload that will turn off the pathway,” he says. Plans for future approaches also include genome editing. Now that the research team had demonstrated proof of concept in preclinical experiments, their hope is to actualize this in the clinical arena. “By dissecting the tumors surgically removed from patients, we could isolate antigens that are ovarian cancer-specific,” he says. “At the same time, we would obtain peripheral blood and differentiate the monocytes in vitro into dendritic cells. If dendritic cells do not present antigens to the T cells, the T cells cannot kill the tumor. So what we want to do exploiting new genome-editing tools is delete XBP1 or IRE1α in the lab and then transfer these gene-modified or gene-edited dendritic cells back into the patient. The process is called adoptive immunotherapy. Our hypothesis is that dendritic cells that no longer have XBP1 or IRE1α would function better in the cancer host.” Closing in on Clinical Applications Eloise Chapman-Davis, MD, is a specialist in gynecological cancers in the Department of Obstetrics and Gynecology at NewYork-Presbyterian/Weill Cornell Medical Center. Her passion in bringing advanced medical care to the underserved around the world and patients at home complements Dr. Cubillos-Ruiz’s aspirations to translate his work at the bench into clinical applications, ultimately providing treatment options for ovarian cancer in an area of oncology that to date has had little reason for optimism. Drs. Cubillos-Ruiz and Chapman-Davis, joined by pathologist Cathleen Matrai, MD, were recently awarded a grant by Colleen’s Dream Foundation to define the role of XBP1 as a potentially crucial biomarker in the treatment of ovarian cancer tumors. Their research builds on their discovery that IRE1α-XBP1 signaling plays a crucial tumorigenic and immunosuppressive role in ovarian cancer. The investigators will analyze tumor specimens from 200 ovarian cancer patients in a retrospective study, quantifying the expression levels of XBP1 in each of the patient’s tumor samples, then correlating their results to existing patient data with the aim of understanding the protein’s effect in disease progression and therapy engagement. They also hope to establish a new system that reveals which patients are better candidates to receive therapies that target XBP1 and other biomarkers. “Our goal is to look for biomarkers that will be able to help predict response or resistance to treatment with standard cytotoxic drugs,” notes Dr. Chapman-Davis. “The ultimate goal is to be able to create an immune-based scoring system to identify specific patients more likely to be affected. And then, hopefully, we will be able to utilize them to direct treatment with immunotherapy in conjunction with conventional chemotherapeutics.” Dr. Chapman-Davis emphasizes that the big unknown is identifying which patients with ovarian cancer are going to be the most receptive to the new immunotherapies that are becoming available. “Our goal is to determine novel immunotherapeutic approaches for treating ovarian cancer, but before we can utilize some of those novel approaches, we need to know which patients will benefit,” she says. “The problem with ovarian cancer is that while we do a good job of getting patients up-front therapies that can put them into remission, the majority will have a recurrence in a short period of time. And, once they recur, you basically lose your chance for a cure. We’re trying to change ovarian cancer into a chronic disease rather than a death sentence.” The challenge is further complicated by preexisting levels of immune dysfunction in patients who are already undergoing treatment for ovarian cancer. “There is a lot of data looking at ways to evaluate T-cell function, T-cell regulation, and tumor infiltration patterns,” notes Dr. Chapman-Davis. “Through this retrospective study, we are trying to see if we can develop a pattern in patients who show variance of expression of XBP1 and link that to their clinical data. Identifying specific features about a particular patient’s immune profile may help direct patient treatment options and increase the chances that a patient would accept the immunotherapy.”
Immunotherapy: A New Frontier in Genitourinary Cancers
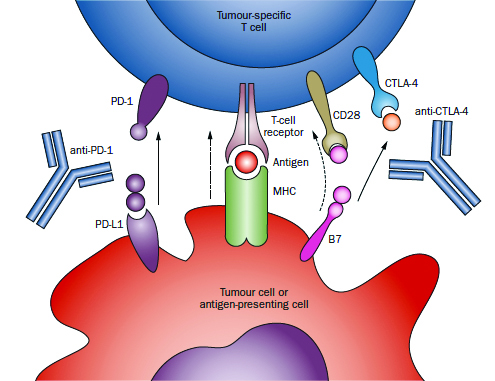
In November 2016, Charles G. Drake, MD, PhD, joined NewYork-Presbyterian/Columbia University Medical Center as the new Director of Genitourinary Oncology. He also serves as Co-Director of Columbia’s Cancer Immunotherapy program along with Naiyer Rizvi, MD, Director of Thoracic Oncology at Columbia. “I was presented with a unique opportunity to build a genitourinary cancer group with the focus on trying to cure these cancers with the immune system, and doing so among a group of clinicians and scientists who, like Dr. Rizvi, are tops in the immunotherapy world.” Dr. Drake emphasizes that his attraction to joining Columbia is the leadership of Stephen G. Emerson, MD, PhD, Director, and Gary K. Schwartz, MD, Deputy Director, of the NCI-designated Herbert Irving Comprehensive Cancer Center, where Dr. Drake also now serves as Associate Director for Clinical Research. “They have been shaping a progressive and comprehensive cancer program in which one of the cornerstones of their vision is the conviction that the immune system can produce meaningful and long-lasting clinical responses in cancer patients. This is what I’ve believed in for a long time and have focused on in my work for nearly two decades.” A physician-scientist, Dr. Drake has established a career that encompasses both the laboratory and the clinic. His work spans the continuum of research from seeking understanding of basic immunological mechanisms to using animal models to understand how drugs work on certain molecules to evaluating immuno-based therapies in clinical trials. After receiving a B.S. in engineering from Rutgers University College of Engineering and a master’s degree in bioengineering at Rutgers, Dr. Drake then pursued his PhD in immunology at the National Jewish Center for Immunology and Respiratory Medicine/University of Colorado Health Sciences Center. Two years later, he completed his MD there. A residency and medical oncology fellowship followed at Johns Hopkins University, where Dr. Drake joined the faculty, rising to become Professor in Oncology, Urology, and Immunology and Co-Director of the Cancer Immunology Program, while continuing incisive research in immunology that had begun during his graduate studies. In more than 80 articles and book chapters, and with many inventions and patents, Dr. Drake has advanced the use of immunotherapies in combination with existing treatments, both surgical and nonsurgical, and across tumor types, including those at advanced stages. Extending the Boundaries of Immunotherapy “Immunotherapy represents a new frontier in genitourinary cancers,” says Dr. Drake. “We’re looking at how it can be used synergistically with traditional therapies in prostate cancer as well as in other tumor types.” Revealing a promising and growing understanding of the mechanisms that encourage the immune system to ignore malignant invaders, Dr. Drake has been a leading voice for the efficacy of immunotherapy. He has been a lead author or integral participant in lauded research and review articles that underscore immunotherapy’s re-emerging potential. These include “Prostate Cancer as a Model for Tumor Immunotherapy,” published in Nature Reviews Immunology; “Breathing New Life Into Immunotherapy: Review of Melanoma, Lung and Kidney Cancer,” published in Nature Reviews Clinical Oncology; “New Strategies in Bladder Cancer: A Second Coming for Immunotherapy,” published in Clinical Cancer Research; and “Recent Advances in Immunotherapy for Kidney Cancer,” published in Discovery Medicine. Early on in his career at Johns Hopkins, Dr. Drake developed a novel transgenic model of prostate cancer in which a unique antigen is expressed exclusively in the prostate gland and in prostate tumors. That model made it possible for researchers to validate that androgen ablation, the most common treatment for progressive prostate cancer, can break tolerance to the prostate gland, lending itself to anti-tumor vaccination. “My team worked very hard to gather immune cells that are infiltrating different kinds of genitourinary cancers,” says Dr. Drake. “We’ve gotten immune cells from prostate, kidney, and bladder cancers, and we’ve studied them very, very carefully using a technique called RNA-Seq that tells us about every single gene and how it’s expressed, and actually how it’s spliced, too. It’s absolutely fascinating.” His research group also discovered several new molecules that made it into the clinic. “The one that is the farthest along is a molecule called LAG-3 [lymphocyte activation gene-3],” notes Dr. Drake. “We showed that LAG-3 could restore anti-tumor immune responses and that blocking multiple immune checkpoints might be required to restore anti-tumor immunity. This molecule is now in Phase I trials in multiple tumor types.” Dr. Drake notes that for a long time it was thought that patients diagnosed with different genitourinary cancers, particularly prostate and kidney cancers, should be rushed into surgery. For example, in patients with high-grade kidney cancer, surgery will cure many of those patients, 60 to 70 percent, but not all. “Many studies have now shown that delaying surgery four to six weeks, particularly if patients are undergoing active treatment, is not clinically significant.” In a recent trial initiated by Dr. Drake at Hopkins, selected patients determined to be at a high risk for a return of the cancer following surgery were given three doses of the immunotherapy drug anti-PD-1 two weeks apart prior to undergoing surgery. “The beauty of this from the patients’ point of view is that this treatment approach may reduce the chances or recurrence,” says Dr. Drake. “This is also, of course, a plus for clinicians, but additionally, at the time of surgery we have the entire kidney tumor available for testing. By analyzing that specimen carefully, we can get a good idea of what a particular immune drug does or doesn’t do. Does it fix the CD8 cells or regulatory T cells? Does the therapy address other populations of immune cells that might be important? From a scientific standpoint, there’s no better way to understand what these drugs do in patients in general, but also, tellingly, in particular patients. We’re learning a lot already from that small trial.” In a trial with prostate cancer, Dr. Drake and his team tested hormonal therapy versus hormonal therapy and a vaccine. “The vaccine has been around for a long time,” Dr. Drake explains, “and it was fascinating to see that the hormonal therapy did a nice job of driving activated CD8 cells into the prostate gland. It was statistically significant, it was tolerated, and it was a really fascinating result. We’re still analyzing all the tissue, but are finding that the vaccine didn’t really add all that much. By doing these neoadjuvant trials, we can accelerate the development of new agents or new combinations of agents. Once we have a good signal in this early stage, we can take the agents to the later stages to look for more obvious signs of clinical activity or hopefully clinical benefit.” At Columbia, Dr. Drake continues his groundbreaking research and is excited about drawing on the extensive expertise and progressive research underway here by cancer specialists and molecular biologists across disciplines. “We want to establish collaborative tumor specific working groups in prostate, kidney, and bladder cancers, with a particular focus on crafting a clinical trials portfolio focused on later stage diseases,” says Dr. Drake. “The goal is to have cutting-edge clinical trials across the diseases and then across the spectrum of diseases. From the very beginning, we want to develop trials where in addition to standard radiation or surgery, we give patients a drug to activate the immune system so that the conventional treatment is more likely to lead to a long-term response without the cancer coming back.”
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||